Since the initial introduction of adhesives by Buonocore, clinical dentistry has progressed significantly due to recent advances in materials science [1]. The bonding systems, with their various generations, now offer a range of products specifically tailored to each clinical scenario. The conventional bonding practice involves etching the enamel, followed by priming the enamel surface, and finally applying the orthodontic adhesive between the enamel and the bracket base. The average optimum SBS of orthodontic adhesives ranges from 8-12 MPa, which is strong enough to ensure adequate retention of brackets and to withstand masticatory and orthodontic forces, while also allowing for easy removal without causing enamel loss during debonding [2].
The conventional bonding practice employs primer-based adhesives. Primers are unfilled resins that contain both hydrophilic and hydrophobic moieties [3]. These primers are reported to facilitate mechanical interlocking between detached enamel prisms and light-polymerised adhesives, leading to adequate bond strength [4,5]. However, the priming step increases the clinical bonding time, which, in turn, is associated with a greater propensity for moisture contamination and subsequent bond failure [6]. Additionally, the use of primers by the operator can lead to hypersensitivity reactions and contact dermatitis [7-9].
The introduction of SCA offers a wide range of advantages, such as increased working time and reduced bonding time by 1-2 minutes during the 5-5 bonding protocol [10,11]. These adhesives contain phosphoric ester monomers, which contribute to a stable bond, thereby eliminating the need for a primer [12]. Cytotoxicity and biocompatibility remain two of the most crucial characteristics of dental materials. Claims of allergic dermatitis and oestrogenicity due to dental monomers have long been debated. Therefore, it is of prime importance to study the cytotoxicity of commercially available orthodontic materials.
SEM experiments measuring the depth of penetration of resin tags have revealed in several studies that the unfilled resin in conventional adhesives is not a requisite for filling the micropores in the etched enamel surface; therefore, a resin phase with filler particles is sufficient to create adequate bond strength [5,13-15]. Three adhesives tested-Enlight, Transbond XT (both Bis-GMA-based orthodontic adhesives), and U Bond (a light-cured self-etch adhesive)-are conventional primer-based adhesives. In contrast, SCA, Aqualine LC, is a HEMA-based, self-priming adhesive, and since it does not require a separate priming step, it reduces chair-side time for operators. Currently, there are no studies comparing this self-priming adhesive with other adhesives.
Therefore, the aim of this study was to assess the cytotoxicity, ARI, and SBS of the self-priming adhesive (Aqualine LC) in comparison to the other three conventional orthodontic adhesives used in the study. Accor ding to the null hypothesis, no discernible variation exists in SBS, ARI, or cytotoxicity among these adhesives. The alternative hypothesis states that there is a significant difference in SBS, ARI, and cytotoxicity among the adhesives.
Materials and Methods
This in-vitro study was conducted in an institutional setting as a collaborative effort between the Department of Orthodontics and White Lab-Blue Lab at Saveetha Dental College and Hospital, Chennai, India, from July 2023 to August 2023. The study was reviewed and approved by the Institutional Human Ethical Clearance Committee, with the reference number IHEC/SDC/ORTHO-2104/23/116.
Eligibility criteria: This study employed forty previously extracted human premolar teeth, which were free of any carious lesions or enamel defects. These teeth were extracted from healthy subjects without any systemic conditions, as part of therapeutic extractions in individuals undergoing orthodontic treatment.
The power of the study was set at 0.95, and the alpha error was set at 0.05. The sample size was estimated using G Power software (Version 3.0.10, Kiel, Germany) with a 1:1 allocation ratio, based on the study by Taubmann A et al., [16]. The sample size was determined to be N=40 (n=10/group). The manuscript was written in accordance with the CRIS guidelines for reporting in-vitro studies [17].
The parameters assessed in this study included SBS, ARI, and the cytotoxicity of the four orthodontic adhesives evaluated.
Specimen Preparation for Testing SBS
The orthodontic adhesives used in this study were categorised into four groups (n=10/group): Group 1-U Bond LC (Vericom); Group 2-Enlight (Ormco); Group 3-Transbond XT (3M Unitek); Group 4-Aqualine LC (Tomy, SCA). [Table/Fig-1] depicts the composition and details of the orthodontic adhesives used in this study. Forty previously extracted human premolar teeth, devoid of any carious lesions or enamel defects, were procured and cleaned in an ultrasonic bath. Each sample was mounted on acrylic blocks after meticulous pumice polishing to remove any surface debris [Table/Fig-2]. Metal brackets (0.22 MBT, American Orthodontics, USA) were bonded to the extracted teeth using a regular orthodontic bonding protocol, in which the extracted teeth were etched for approximately 15-20 seconds using 37% orthophosphoric acid (D-Tech etching gel, India), followed by a thorough rinse and air-drying process until a frosty white appearance was observed. Using an LED light curing unit (Ivoclar Bluephase Powercure, Zurich) with a light intensity of 2,000 mW/cm2, the appropriate bonding agent for each adhesive system was applied to all samples for five seconds for priming, followed by the application of the above-mentioned primer-based adhesives, which were light-cured for 10 seconds on each proximal side. For Group 4, using the Aqualine LC adhesive, the brackets were etched and dried, and each proximal side was then light-cured for 10 seconds. To test for SBS, a Universal Testing Machine (UTM) (Instron 4465, Canton, Massachusetts) was utilised, with a speed of 1 mm/min. At the bracket-tooth interface, a shear load was applied by a plunger exerting an occluso-gingival load on the brackets. The maximum recorded loads in kilograms were converted to MPa (nominal surface area: 9.91 mm2). [Table/Fig-3] illustrates the experimental set-up for the SBS test.
Characteristics of the tested adhesives.
| S. No. | Group | Adhesive | Manufacturer |
|---|
| 1. | Group 1 | U-Bond LC (UB) | Vericom, Gangwon-do, South Korea |
| 2. | Group 2 | Enlight (E) | Ormco, USA |
| 3. | Group 3 | Transbond XT (TB) | 3M Unitek, CA, USA |
| 4. | Group 4 | Aqualine LC (Aq) | Tomy, Tokyo, Japan |
Sample preparation for SBS.
Demonstrates the experimental set up to test SBS using Instron UTM.

Adhesive Remnant Index (ARI): After subjecting the samples to the SBS test, they were evaluated for ARI, originally proposed by Artun and Bergland, through SEM analysis [18]. The ARI index is a graded score indicative of the type of bond failure:
0-No composite left on the tooth;
1-Less than 50% composite left on the tooth;
2-More than 50% composite left on the tooth;
3-Entire composite left on the tooth with a clear impression of the bracket base.
The same observer (HN) graded the SEM images of all specimens. Additionally, the same observer evaluated three randomly selected specimens for ARI after one week, and intra-observer reliability was subsequently calculated.
Sample Preparation for Cytotoxicity Test
Each adhesive tested in the present study was meticulously packed into a spherical plastic template and pressed with a glass slide. The adhesive was then light-cured using an LED light cure unit for 10 seconds to produce spherical pellets with a diameter of 3 mm, which were carefully retrieved from the template molds. These pellets were subsequently used for the MTT (3-{4,5-dimethylthiazol-2-yl}-2,5-diphenyl tetrazolium bromide) assay to test cytotoxicity [Table/Fig-4].
Illustrates spherical pellets prepared for testing cytotoxicity.
Human Gingival Fibroblast Cells (HGFC): HGFC cells were cultured in Eagle’s minimum essential medium F12, containing 15% (vol/vol) heat-inactivated Foetal Bovine Serum (FBS), 2 mM L-glutamine, 50 IU/mL penicillin, and 50 mg/mL streptomycin. Standard incubation conditions (37°C, 95% air/5% CO2) were used to grow the cells in T-25 cm2 culture flasks until confluence (approximately 70%-80%). After one week, the cells were dissociated using a trypsin solution and then replated in 6-well plates at a cell density of 2.5 x 105 cells per well. Two milliliters of complete DMEM F-12 (Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12) medium were added to each well, and after 24 hours, the cells were allowed to attach [19].
MTT Assay for Cytotoxicity Assessment
The bottom well of a 6-well plate, containing 1 mL of full culture medium and ground adhesive pellets, was supplemented with 0.5 mg/mL MTT, followed by incubation of the plate at 37°C for four hours [Table/Fig-5]. After the incubation period, the culture medium was removed from the insert well, and 100 μL of DMSO (Dimethyl sulfoxide) solution was added to each well to solubilise the resulting formazan crystals. To ensure homogeneous mixing of the solvent and the blue reaction product, the cells were gently shaken for two minutes. Lastly, to quantify cell viability, 100 μL of the coloured DMSO was transferred from each insert and each well to a new 96-well plate. A microplate reader was used to measure the absorbance at 570 nm. The relative viability of the cells (CV) in comparison to the control (an empty well) was calculated using the following formula:
Six well plate before testing for Optical Density (OD).
% Cell Viability={OD570 of treated cells}×100%/{OD570 of control cells}.
The method outlined by Sjogren et al., was used to grade cell viability [20], wherein:
>90% cell viability=non-cytotoxic
60-90% CV=mildly to slightly cytotoxic
35-59%=moderately cytotoxic
<30%=severely cytotoxic.
Statistical Analysis
SPSS software (Version 23.0) was used to conduct the statistical tests. The Shapiro-Wilk test was used to determine the normality of the data. Kappa statistics were utilised to assess intra-observer reliability. A significance threshold of p>0.05 was set. The Analysis of Variance (One-way ANOVA) test was conducted to evaluate the inter group SBS values, followed by Post hoc Tukey’s test for multiple-level alpha control. The Kruskal-Wallis test was used to assess inter group ARI and cytotoxicity. A p-value <0.005 was considered significant.
Results
Shear Bond Strength (SBS)
The values for SBS were measured in megapascals (MPa). The data exhibited a normal distribution when tested with the Shapiro-Wilk test. Inter group comparisons of SBS revealed a significant difference (p<0.001) through the one-way ANOVA test [Table/Fig-6]. Pairwise inter group comparisons using post-hoc Tukey’s test showed significant differences between Group 1 (EnlightTM) and the other three groups (p<0.001). The highest SBS was obtained for Group 3 (Transbond XTTM), while the lowest SBS was observed for Group 1 (EnlightTM). A comparative evaluation of SBS values among the four groups revealed a statistically significant difference (p-value <0.001) [Table/Fig-7].
Mean SBS values of the tested adhesives.
| Group | Mean | Std. Dev. | Sig. |
|---|
| 1 | 7.495 | 1.395 | <0.001* |
| 2 | 5.445 | 0.521 |
| 3 | 8.277 | 0.772 |
| 4 | 8.123 | 1.068 |
Tukey HSD test for pairwise intergroup comparisons.
| Group | Pairwise comparison | Sig. |
|---|
| 1 | 2 | <0.001* |
| 3 | 0.309 |
| 4 | 0.500 |
| 2 | 1 | <0.001* |
| 3 | <0.001* |
| 4 | <0.001* |
| 3 | 1 | 0.309 |
| 2 | <0.001* |
| 4 | 0.985 |
| 4 | 1 | 0.500 |
| 2 | <0.001* |
| 3 | 0.985 |
Adhesive Remnant Index (ARI)
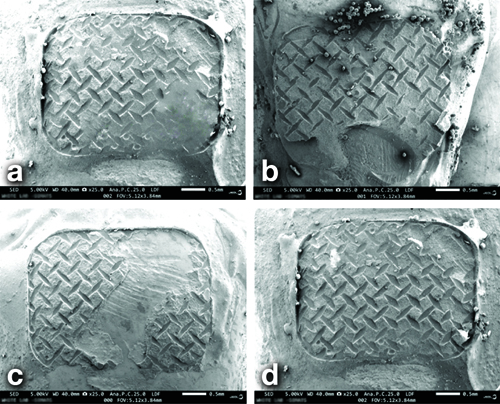
The kappa statistic for intra-observer reliability showed k=0.78, which suggests substantial agreement. [Table/Fig-8a-d] depicts the SEM analysis images of the four adhesives tested in this study. The Kruskal-Wallis test reveals a significant difference in pairwise comparisons, as indicated by Dunn’s test (p<0.001) and Chi-square (χ2)=27.596, therefore implying the rejection of the null hypothesis. [Table/Fig-9] depicts the descriptive statistics of the ARI observed for the different adhesive groups tested.
SEM images after debonding for ARI assessment. (a) ARI 1; b) ARI 2; c) ARI 2; d) ARI 3).
Descriptive statistics of ARI values measured for the different adhesives.
| ARI score | Group 1 (%) | Group 2 (%) | Group 3 (%) | Group 4 (%) | p value |
|---|
| 0 | 0 (0) | 0 (0) | 2 (20%) | 0 (0) | <0.001* |
| 1 | 0 (0) | 0 (0) | 5 (50%) | 7 (70%) |
| 2 | 3 (30%) | 7 (70%) | 3 (30%) | 2 (20%) |
| 3 | 7 (70%) | 3 (30%) | 0 (0) | 1 (10%) |
Cytotoxicity Test
The samples were subjected to a cytotoxicity test using the MTT HGFC cell assay, and the graphical representation is shown in [Table/Fig-10]. No significant difference (p-value=0.534) was noted in the inter group comparison, and all adhesives demonstrated acceptable levels of biocompatibility, although a slight increase in mitotic activity was observed. When ranking cell viability levels using the Kruskal-Wallis test, the least cytotoxicity was shown by Enlight, as listed in [Table/Fig-11], however it was not statistically significant. Comparable cytotoxicity levels were observed in Aqualine LC, U-Bond, and Transbond XT.
Graphical representation of % cell viability of the tested adhesives.
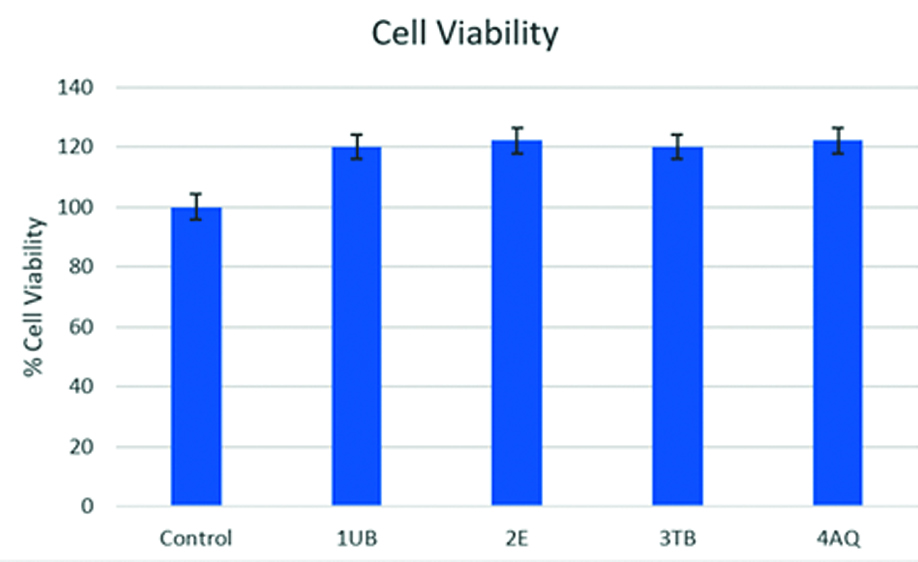
Intergroup comparison for cytotoxicity.
| S. No. | Group | Group average | SD | % Cell viability | Mean rank | p-value |
|---|
| 1 | Control | 0.14 | 0.035279 | 100 | 50.17 | 0.534 |
| 2 | U Bond | 0.12 | 0.025598 | 120.0825 | 27.38 |
| 3 | Enlight | 0.11 | 0.025314 | 122.1833 | 23.13 |
| 4 | Transbond | 0.11 | 0.021642 | 119.1833 | 24.71 |
| 5 | Aqualine LC | 0.12 | 0.024351 | 121.03356 | 25.13 |
Kruskal Wallis Test
Discussion
Biomaterials play an important role in achieving successful orthodontic treatment outcomes. An ideal bonding material must exhibit adequate SBS after bonding and be biocompatible with oral tissues, without causing any hypersensitivity reactions, while also reducing clinical bonding time. There is limited research on SBS and ARI and Cytotoxicity www.jcdr.net evaluations of single-component adhesives (Aqualine LC) compared to conventional primer-based adhesives (UBond, Enlight, Transbond XT) [10,21]. This in-vitro study aimed to contribute to the existing literature by conducting a comparative evaluation of the SBS, ARI, and cytotoxicity of commercially available orthodontic adhesives used in clinical practice.
The results of this study suggested that there was a significant difference in SBS values among the different adhesives tested, with Group 2 (Enlight™) exhibiting the lowest SBS, while the other three adhesives reported similar SBS values. Inter group comparisons for ARI values revealed significant differences among the various groups, with Group 3 (Transbond XT) showing the lowest ARI and Group 2 (Enlight) demonstrating the highest ARI values. No significant differences in cytotoxic activity among the different adhesives were noted.
In clinical practice, the optimum SBS for orthodontic adhesive is reported to range from 5.9 to 7.8 MPa [22]. This range not only ensures adequate retention of orthodontic brackets on the enamel surface but also remains optimal during debonding, without causing harmful adverse effects to the enamel [23]. The common causes of clinical bond failure in the first six months have been attributed to technical sensitivity, occlusal forces, and the consumption of restricted foods during the course of treatment. Various studies have tested the SBS of different adhesives. The mean and standard deviation of SBS values of the orthodontic adhesives tested in the present study were higher compared to similar studies. In the present study, brackets bonded with Transbond XT exhibited the highest SBS, followed by the Aqualine LC group and the U-Bond group. These findings align with those of Shalini S et al., who reported the highest SBS for Transbond XT and the lowest for a low viscosity self-curing adhesive, Heliosit (Ivoclar Vivadent, Liechtenstein) [24]. Similar results were also reported in another comparative study by Rai S et al., which concluded that Heliosit and Orthofix adhesives had the lowest SBS values, while Transbond XT had the highest SBS values [25]. The brackets bonded with Enlight adhesive showed the lowest SBS values in the current investigation. Additionally, when comparing SCA with other conventional primer-based adhesives, i.e., Aqualine LC and Transbond XT, no significant differences were noted. An in-vitro performance study and a randomised controlled clinical trial of SCA (Biofix and GC Ortho Connect), comparing it with a conventional primer-based adhesive (Transbond XT), conducted by Ok U et al., reported no significant difference in bond failures between the two groups during an observation period of 12 weeks [10]. This study also noted a difference in interlocking tags between the SCA and conventional groups when viewed under scanning electron microscopy (SEM), revealing adequate mechanical interlocking in the SCA group and suggesting that a resin primer was not necessary to achieve optimal bond strength. In accordance with these findings, another in-vitro study by Ferguson JW et al., reported that the primer did not significantly influence bond strength [26]. SCAs have been reported to have significantly shorter clinical bonding times (1.32 minutes), which is attributed to their viscosity. Additionally, there is a reduction in cost and inventory, making them preferable over primer-based adhesives [10].
The ARI scores of the different adhesives tested indicated that the lowest scores were noted in the Transbond XT group. Therefore, a slightly lower risk of bond failure at the enamel-adhesive interface could be anticipated when compared to the SCA group. This finding is consistent with the study results by Ok U et al., and Griffin J et al., who also reported lesser ARI scores for the Transbond group, similar to those of the present study [10,27]. In contrast to these findings, other in-vitro studies have reported greater adhesive left on the enamel surface when using total-etch systems [25,28,29]. However, the SCA group in this study did not show any enamel microcracks when viewed under SEM, which is typically associated with lower ARI scores. Lesser ARI scores are also clinically correlated with reduced polishing time, resulting in less enamel loss. Lower ARI scores are desirable as they imply lesser stress concentrations at the enamel-adhesive interface during debonding, thereby reducing the risk of enamel fracture [30]. However, the ARI results should be interpreted with caution since it is a subjective scale; therefore, a volumetric analysis of remnants on microtomography immediately after debonding would provide more substantial evidence [31]. Since this was an in-vitro study, in-vivo correlation of the study findings is needed.
Cytotoxicity has been attributed to unreacted residual polymers, primarily Bisphenol A. Among the dimethacrylate derivatives, Bis-GMA is reported to be the most potent toxic component [32]. In this study, the effect of four different adhesives was investigated for the first 24 hours using the MTT assay on HGFC. The results revealed that all the adhesives were non-cytotoxic, with no significant difference between the groups. However, the percentage cell viability values of the tested adhesives were slightly greater than those of the control group, suggesting increased mitotic cell proliferation. Studies also report that unreacted monomer compounds may alter routine cell functioning, which was noted due to elevated p53 and p63 protein values in a study that depicted the genotoxic effects of some adhesives, according to an immunohistochemical study by Angiero F et al., [33]. In another recent study, it was reported that the tested light-cured adhesives (Transbond XT, Transbond LR, Filtek Supreme XTE, Clearfill) were slightly cytotoxic to Gingival Fibroblast (GF) cells due to the leaching of monomers like BPA and TEGDMA, contrary to the present study findings, where all the tested adhesives exhibited no cytotoxicity [34]. A systematic review reporting on in-vitro cytotoxicity of dental adhesives indicated conflicting results, with an overall greater cytotoxicity for etch-and-rinse adhesives, and significant heterogeneity in the experimental protocols of the included studies [35].
Limitation(s)
The SBS determination depends on many factors, and studies enlist the following machine testing factors that could be influential in an in-vitro setting: force magnitude and its application, direction, angulation, and location [35]. Finite element analysis studies also report non-homogeneous stresses near the adhesive-enamel interface, as well as, in the alveolar bone and periodontium during testing [35]. Therefore, the study findings must be interpreted with caution, as in-vitro activities do not completely replicate conditions in the oral environment.
Conclusion(s)
Significant differences in SBS were noted among the groups, with Enlight exhibiting the lowest SBS and Transbond XT the highest. Comparable SBS values were observed between SCA (Aqualine LC) and other conventional primer-based adhesives. Significant differences in ARI scores among the different groups were noted; however, ARI scores between Transbond XT and Aqualine LC showed no significant differences. All adhesives tested demonstrated acceptable levels of biocompatibility. The research advocates for clinicians to adopt SCA in their procedures, citing similar bond strengths, ARI, and cytotoxicity to traditional primer-based adhesives. This shift promises increased ease for the operator and reduced chairside time.