Introduction
Sepsis is a clinical condition that results from infection and systemic inflammatory response syndrome, which can progress to severe sepsis and septic shock. This condition leads to myocardial dysfunction and increased mortality rates, particularly in cases of septic shock. Inotropic agents are beneficial in improving cardiac contractility and cardiac output in septic shock.
Need of the study
It is important to address myocardial dysfunction in sepsis and septic shock, as these conditions significantly contribute to patient morbidity and mortality. Calcium desensitisation is a key factor in the pathophysiology of septic myocardial depression, leading to impaired cardiac function. Levosimendan, a novel calcium sensitiser, offers a promising therapeutic option due to its unique pharmacologic and biologic profile. By enhancing calcium sensitivity in cardiac myocytes, levosimendan may improve cardiac function without the detrimental side-effects associated with traditional inotropic agents.
Aim
The research protocol was planned with an aim to analyse the impact of intravenous levosimendan on Right Ventricular (RV) dysfunction in patients with septic shock.
Materials and Methods
This prospective observational study aims to assess the effect of levosimendan on the improvement of RV dysfunction in patients with septic shock who are admitted to the intensive care unit of Acharya Vinoba Bhave Rural Hospital, DMIHER (DU), Sawangi (Meghe), Wardha, Maharashtra, India from July 2023 to March 2025, 45 patients who meet the inclusion criteria will be recruited. Transthoracic Echocardiography (TTE) will be performed using standard measures to calculate RV function. Patients diagnosed with RV dysfunction will be administered levosimendan, followed by a re-evaluation of RV function at designated intervals. Statistical analysis will be conducted using R software, and a paired t-test will be employed to determine significant differences at pre- and post-intervention timelines for the outcome variables of echocardiographic parameters, including Tricuspid Annular Plane Systolic Excursion (TAPSE), EF, RV Fractional Area Change (FAC), and Tricuspid Regurgitant jet Velocity (TRV), at a 5% level of significance.
Introduction
Sepsis is a clinical condition resulting from infection and systemic inflammatory response syndrome, which can progress to severe sepsis and septic shock [1,2]. In the European study “The Sepsis Occurrence in Acutely Ill Patients”, it was reported that more than thirty-five percent of ICU patients experienced sepsis during their hospital stay, with an associated death rate of twenty-seven percent [3]. Both gram-positive and gram-negative micro-organisms can cause sepsis and septic shock in the ICU. The most common infections in this setting include pneumonia, urinary tract infections, abdominal infections, Central Line-Associated Bloodstream Infections (CLABSI), and skin and soft-tissue infections [4,5]. In an Indian study, the death rate of patients with severe sepsis was reported to be 85% [6].
The clinical manifestations of sepsis and septic shock lead to the activation of pro-inflammatory and anti-inflammatory mediators, which subsequently disrupt mitochondrial function, microcirculatory flow, and coagulation pathways, ultimately resulting in multiple organ system failure.
The heart is the primary organ affected by sepsis. It impacts myocardial function, vascular tone, and capillary integrity. Inflammatory mediators such as cytokines, Tumour Necrosis Factor-alpha (TNF-alpha), Interleukin-2 (IL-2), and IL-6 may exert a direct myocardial depressant effect. Cytokines are involved in reflex tachycardia, reduced contractility, diffuse capillary leaks, and peripheral arterial dilation. Patients typically present with lower mean arterial pressure and diastolic hypotension. Sepsis-related myocardial dysfunction is characterised by reduced cardiac output, diastolic dysfunction, decreased EF, and biventricular dilation [7]. The RV dysfunction is linked to a higher 28-day mortality rate and is the most prevalent manifestation in individuals with sepsis and septic shock. RV failure may hinder Left Ventricular (LV) filling, ultimately decreasing cardiac output due to ventricular interdependence [8,9]. RV dysfunction is observed in almost thirty to sixty percent of all patients with sepsis and septic shock and is often associated with LV dysfunction [10,11].
In response to myocardial dysfunction in septic shock, specific interventions are required, with levosimendan playing a key role. Given its unique mechanism of action, which involves calcium sensitisation and potassium channel opening, levosimendan provides positive inotropic effects without increasing myocardial oxygen demand [12,13]. Its vasodilatory effect is achieved by opening Adenosine Triphosphate (ATP)- dependent potassium channels in vascular smooth muscle cells, resulting in a reduction in RV preload and afterload [14-16]. This may be beneficial in the context of septic shock-induced RV dysfunction [17]. In practice, intravenous levosimendan is infused at a rate of 0.1-0.2 mcg/kg/minute for a maximum of 24 hours after an initial loading dose of 6-12 mcg/kg [18]. The null hypothesis considered for the planned study was there will be no significant difference in echocardiographic parameters before and after drug administration.
The research protocol was planned to analyse the impact of intravenous levosimendan on RV dysfunction in patients with septic shock.
Primary objective: To evaluate the functioning of the right ventricle in patients with septic shock using TTE.
Secondary objectives: To ensure adequate fluid resuscitation therapy for patients with septic shock, the authors will check the inferior vena cava compressibility index and adhere to the one-hour bundle of the Surviving Sepsis Guidelines.
To administer an intravenous Levosimendan infusion with a loading dose of 6 mcg/kg followed by a continuous infusion of 0.1-0.2 mcg/kg/min for a maximum of 24 hours, to patients with septic shock who exhibit RV failure.
To assess the improvement in RV function after 48 hours and seven days of drug administration.
Review of Literature
In a study conducted by Angelos Arfaras-Melainis, it was noted that there is a gap in knowledge in the field of heart failure due to sepsis, indicating that further studies are required to understand the choice of inotropic agents [19]. In a randomised controlled study by Morelli A et al., it was found that levosimendan improved sublingual microcirculatory blood flow in patients with septic shock [20]. However, a study by Gordon AC et al., revealed that adding levosimendan to the treatment of patients with sepsis or septic shock did not provide any mortality benefit and was associated with tachycardia and supraventricular arrhythmia [21].
A systematic review and meta-analysis conducted by Liu D-H et al., showed that patients with sepsis and septic shock experienced improvements in cardiac index and reductions in lactate levels after 24 hours of levosimendan administration, but there was no associated mortality benefit [22].
In a prospective, randomised, placebo-controlled pilot study involving 35 patients with ARDS and septic shock treated in a university hospital’s ICU, participants received either a 24-hour infusion of levosimendan (n=18) or a placebo (n=17). Data were collected from various cardiovascular measurements before and after treatment. The results indicated that levosimendan increased cardiac index, reduced mean pulmonary artery pressure and pulmonary vascular resistance, and improved RV EF while lowering RV end-systolic volume. Additionally, mixed venous oxygen saturation rose significantly with levosimendan. These findings suggest that levosimendan enhances RV performance through pulmonary vasodilation. To confirm these benefits and assess overall prognosis improvement, a larger multicenter trial is necessary [23].
Based on the above studies, the authors aim to further evaluate the efficacy of levosimendan in patients with sepsis and septic shock who have RV dysfunction. As levosimendan is a calcium sensitiser, it can help increase cardiac contractility with a minimal increase in myocardial oxygen consumption. The authors will assess RV dysfunction in sepsis using 2D echocardiography after adequate fluid resuscitation therapy for septic shock patients and will follow a one-hour bundle of the surviving sepsis guidelines. Improvement in RV dysfunction will be assessed after 48 hours and again after seven days of hospitalisation.
Materials and Methods
This research is an observational prospective cross-sectional study aimed at assessing RV function in septic shock patients admitted to Acharya Vinoba Bhave Rural Hospital, DMIHER (DU), Sawangi (Meghe), Wardha, Maharashtra, India. The Institutional Ethics Committee (IEC) reference number is DMIMS(DU)/IEC/2022/298. The duration of the study will be from July 2023 to March 2025.
All prospective patients with RV failure due to septic shock who are admitted to the critical care medicine department will be included in the study after obtaining the patient’s consent.
Inclusion criteria: Those adult patients aged 18 years or older, diagnosed with sepsis and septic shock i.e., systolic blood pressure of less than 90 mmHg, even after a 20 mL/kg intravenous fluid challenge and while on vasopressor support and patients with septic shock who also have RV failure are planned to be included.
Exclusion criteria: Those patients aged less than 18 years, pregnant women, patients with valvular heart disease, patients with LV outflow tract obstruction and those patients undergoing TTE beyond 24 hours from the onset of sepsis or with subpar image quality will be excluded from the study.
All prospective patients with sepsis admitted to the intensive care unit will be included in the study after obtaining the patient’s consent.
Data Collection Process
To ensure the precision and reliability of the data collected for this project, systematic and consistent techniques will be employed throughout the data gathering process. The data collection operations will be conducted by skilled research professionals under the supervision of the lead investigator.
Sample size: Approximately, 45 individuals with RV dysfunction related to septic shock are anticipated to participate in the trial. The sample size was calculated based on the prevalence of isolated RV dysfunction in previous studies, which was found to be 47% [24]. Minimum sample size required:
Formula:
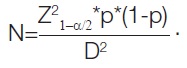
The prevalence of isolated RV dysfunction was 47%
P=47%=0.47
D=estimated error (15%)=0.15
=(1.96)2 * (0.47) * (1- 0.47)/(0.15)2=45
The minimum sample size is 45:
Forty-five patients make up the projected sample size, with an allowable error of 15%. To ensure the research objectives and sample homogeneity, patients diagnosed with RV dysfunction associated with sepsis and septic shock will be included in the study based on the established inclusion criteria.
Demographic variables: The following information will be collected: age, sex, BMI, SBP, DBP, pulse, past medical history, comorbidities, and a history of risk factors, including being over 65 years of age and having chronic illnesses such as HIV and cancer. Outcome variables such as TAPSE, EF, right ventricle FAC, and TRV will also be collected.
Primary outcome: Transthoracic echocardiographic measures: Common echocardiographic measures, including Tricuspid Annulus Plane Systolic Excursion (TAPSE) from M-mode, RV FAC, LVEF, and TRV, will be assessed according to the American Society of Echocardiography guidelines [25]. Strain analysis will utilise standard apical four-chamber images, selecting the highest-quality single cardiac cycle available. Images that do not meet quality standards will be rejected. Fluid status will be assessed by checking the inferior vena cava compressibility index [26].
Secondary outcome: The aim is to reduce the length of ICU stay and associated morbidity. Several secondary outcomes may arise, such as analysing the effects of treatment delays on patient outcomes, gauging the efficacy of initiatives aimed at lowering barriers, and determining ways to elevate the standard of care and patient satisfaction. By examining these secondary outcomes, we can develop targeted solutions to address patients’ issues and gain a comprehensive understanding of their challenges [27].
Study Procedure
TAPSE can be determined using M-mode echocardiography by aligning the lateral tricuspid annulus with the ventricular apex in the apical four-chamber view and measuring the lateral annular displacement. The Ejection Fraction (EF) is calculated by dividing the amount of blood pumped out of the ventricle with each contraction (Stroke Volume, or SV) by the End-Diastolic Volume (EDV), which is the total volume of blood in the ventricle. To express this as a percentage, multiply the result by 100. Thus, EF is represented as (SV/EDV) ×100. Typically measured via echocardiography, the RV FAC provides a two dimensional assessment of the global systolic function of the right ventricle. It is computed as follows, based on data obtained from the apical four-chamber view: (End-Diastolic Area-End-Systolic Area)/End-Diastolic Area, yielding the RV FAC. An abnormal TRV is defined as greater than 2.80 m/sec, as determined by Doppler echocardiography [27]. A TRV value of 2.90-3.40 m/s indicates an intermediate probability of pulmonary hypertension [28].
RV dysfunction will be defined as either a RV FAC of less than 35% or a TAPSE of less than 1.6 cm [29]. LV systolic dysfunction will be defined as an EF of less than 45% or a global longitudinal strain of at least 19% [29].
Intervention: Patients meeting the criteria for RV dysfunction [30] will receive an injection of levosimendan at a loading dose of 6 to 12 micrograms per kilogram of body weight, followed by a continuous infusion of 0.1 to 0.2 micrograms per kilogram per minute for up to 24 hours [31].
Follow-up: The RV function will be reassessed on days 2 and 7 post-intervention using a 2D echocardiogram with the parameters mentioned above.
Data analysis: The data collected will be analysed to determine the outcomes of the entire analysis set, utilising appropriate statistical methods to assess changes in RV function following the intervention. This approach seeks to provide important insights into the treatment of patients with septic shock by thoroughly evaluating RV function.
Data collection: TTE will be conducted using an ESAOTE SPA MyLAB Six machine, following clinical indications. Each TTE will be performed by a certified diagnostic cardiac sonographer, with advanced cardiac sonographers responsible for formatting and interpreting the studies. Consensus interpretation will be provided by two level II echocardiographers.
Transthoracic echocardiographic measures: Common echocardiographic measures, including TAPSE from M-mode, RV FAC, and LVEF, will be assessed according to established guidelines. Strain analysis will utilise standard apical four-chamber images, selecting the highest-quality single cardiac cycle available. Images that fail to meet quality standards will be rejected.
Statistical Analysis
Statistical analysis will be conducted using R software. A paired t-test will be employed to determine the significant differences in echocardiographic parameters at pre- and post-intervention timelines, specifically for TAPSE, EF, RV FAC, and TRV, at a 5% level of significance.
[1]. Lanspa MJ, Cirulis MM, Wiley BM, Olsen TD, Wilson EL, Beesley SJ, Right ventricular dysfunction in early sepsis and septic shockChest 2021 159(3):1055-63. [Google Scholar]
[2]. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conferenceCrit Care Med 2003 31(4):1250-56. [Google Scholar]
[3]. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Sepsis occurrence in acutely ill patients investigators. Sepsis in European intensive care units: Results of the SOAP studyCrit Care Med 2006 34(2):344-53. [Google Scholar]
[4]. Wheeler AP, Bernard GR, Treating patients with severe sepsisN Engl J Med 1999 340(3):207-14. [Google Scholar]
[5]. Martin GS, Mannino DM, Eaton S, Moss M, The epidemiology of sepsis in the United States from 1979 through 2000N Engl J Med 2003 348(16):1546-54. [Google Scholar]
[6]. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitationsAm J Respir Crit Care Med 2016 193(3):259-72. [Google Scholar]
[7]. Flynn A, Chokkalingam Mani B, Mather PJ, Sepsis-induced cardiomyopathy: A review of pathophysiologic mechanismsHeart Fail Rev 2010 15(6):605-11. [Google Scholar]
[8]. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of CardiologyEur J Heart Fail 2016 18(3):226-41. [Google Scholar]
[9]. Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impactIntensive Care Med 2016 42(5):862-70. [Google Scholar]
[10]. Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shockMayo Clin Proc 2012 87(7):620-28. [Google Scholar]
[11]. Landesberg G, Jaffe AS, Gilon D, Levin PD, Goodman S, Abu-Baih A, Troponin elevation in severe sepsis and septic shock: The role of left ventricular diastolic dysfunction and right ventricular dilatationCrit Care Med 2014 42(4):790-800. [Google Scholar]
[12]. Lancaster MK, Cook SJ, The effects of levosimendan on [Ca2+]i in guinea-pig isolated ventricular myocytesEur J Pharmacol 1997 339(1):97-100. [Google Scholar]
[13]. Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Myocardial efficiency during levosimendan infusion in congestive heart failureClinical Pharmacology & Therapeutics 2000 68(5):522-31. [Google Scholar]
[14]. Haikala H, Nissinen E, Etemadzadeh E, Levijoki J, Lindén IB, Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxationJ Cardiovasc Pharmacol 1995 25(5):794-801. [Google Scholar]
[15]. Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N, Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytesEur J Pharmacol 1997 333(2-3):249-59. [Google Scholar]
[16]. Parissis JT, Rafouli-Stergiou P, Paraskevaidis I, Mebazaa A, Levosimendan: From basic science to clinical practiceHeart Fail Rev 2009 14(4):265-75. [Google Scholar]
[17]. Kaheinen P, Pollesello P, Levijoki J, Haikala H, Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channelsJ Cardiovasc Pharmacol 2001 37(4):367-74. [Google Scholar]
[18]. Papp Z, Csapó K, Pollesello P, Haikala H, Édes I, Pharmacological mechanisms contributing to the clinical efficacy of levosimendanCardiovasc Drug Rev 2005 23(1):71-98. [Google Scholar]
[19]. Arfaras-Melainis A, Polyzogopoulou E, Triposkiadis F, Xanthopoulos A, Ikonomidis I, Mebazaa A, Heart failure and sepsis: Practical recommendations for the optimal managementHeart Fail Rev 2020 25(2):183-94. [Google Scholar]
[20]. Morelli A, Donati A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Levosimendan for resuscitating the microcirculation in patients with septic shock: A randomized controlled studyCrit Care 2010 14(6):R232 [Google Scholar]
[21]. Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RM, Santhakumaran S, Levosimendan for the prevention of acute organ dysfunction in sepsisN Engl J Med 2016 375(17):1638-48. [Google Scholar]
[22]. Liu DH, Ning LY, Lei YY, Chen J, Liu YY, Lin FX, Levosimendan versus dobutamine for sepsis-induced cardiac dysfunction: A systematic review and meta-analysisSci Rep 2021 11:2033310.1038/s41598-021-99716-9 [Google Scholar] [CrossRef]
[23]. Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: A pilot studyCrit Care Med 2006 34(9):2287-93. [Google Scholar]
[24]. Vallabhajosyula S, Kumar M, Pandompatam G, Sakhuja A, Kashyap R, Kashani K, Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: An 8-year historical cohort studyAnn Intensive Care 2017 7(1):94 [Google Scholar]
[25]. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of EchocardiographyJ Am Soc Echocardiogr 2010 23(7):685-713.10.1016/j.echo.2010.05.01020620859 [Google Scholar] [CrossRef] [PubMed]
[26]. Ilyas A, Ishtiaq W, Assad S, Ghazanfar H, Mansoor S, Haris M, Correlation of IVC diameter and collapsibility index with central venous pressure in the assessment of intravascular volume in critically ill patientsCureus 2017 9(2):e1025 [Google Scholar]
[27]. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trialThe Lancet 2002 360(9328):196-202. [Google Scholar]
[28]. Fadel BM, Husain A, Alassoussi N, Dahdouh Z, Mohty D, Spectral Doppler of the hepatic veins in pulmonary hypertensionEchocardiography 2015 32(1):170-73. [Google Scholar]
[29]. Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, Assessment of pulmonary artery pressure by echocardiography-A comprehensive reviewInt J Cardiol Heart Vasc 2016 12:45-51. [Google Scholar]
[30]. Wu VC, Takeuchi M, Echocardiographic assessment of right ventricular systolic functionCardiovasc Diagn Ther 2018 8(1):70-79. [Google Scholar]
[31]. Antila S, Sundberg S, Lehtonen LA, Clinical pharmacology of levosimendanClin Pharmacokinet 2007 46(7):535-52. [Google Scholar]