The Male Breast Carcinoma (MBC) is a rare type of carcinoma, accounting for only about 1% of all breast cancer cases. The limited availability of cases has restricted the knowledge and understanding of the pathology of MBC compared to Female Breast Carcinoma (FBC). Generally, MBCs are associated with advanced stages; however, unlike FBC, they are more often positive for Oestrogen Receptors (ER) and Progesterone Receptors (PR) [1,2]. Overall, survival data on MBC remain controversial, and radical surgery followed by chemotherapy and radiotherapy is the preferred treatment option [3].
Recently, Programmed cell Death protein 1 (PD-1) and Programmed Death-ligand 1 (PD-L1) have gained popularity as potential targets for immunotherapy in breast cancers. Immune checkpoint inhibitor trials on Triple-negative Breast Cancers (TNBC) have shown promising results [4]. Expression of PD-1 in tumour-infiltrating immune cells and PD-L1 expression in tumour cells are usually associated with higher tumour grades and poorer prognoses; however, some studies have shown conflicting results [5-7]. Furthermore, the expression of PD-1 and PD-L1 has been shown to vary between different molecular subtypes, with consistent expression of PD-L1 in Triple-negative Breast Cancers (TNBC) compared to non TNBC [8,9]. Given the unique biology of MBC in comparison to FBC, the expression profile of PD-L1 may differ. However, the analysis of PD-L1 expression in Male Breast Cancer (MBC) is quite uncommon in the literature, and thus, the immunostaining expression pattern and any differences from FBC remain elusive [10].
Materials and Methods
This cohort study was conducted in the Pathology Department of IPGMER and SSKM Hospital, Kolkata, West Bengal, India. A total of 204 patients who underwent mastectomy over a study period of four years, from January 2020 to December 2023, were selected. Institutional Ethical Clearance was obtained (IPGME&R/IEC/2020/355). Whenever a postoperative mastectomy specimen of suspected breast carcinoma was received from the Department of Surgery, the particular patient was approached in the surgery ward for necessary consent. After obtaining consent, relevant patient particulars were gathered along with history and documentation from the bed head tickets.
Sample size: Using a feasibility method of non randomised sampling, data for 50 male patients and 154 female patients were included in the study.
Inclusion and Exclusion criteria: Postoperative mastectomy specimens of suspected breast cancer patients (male and female) visting the Department of Surgery, who gave wriiten consent were included in the study. Patients who received neoadjuvant chemotherapy or radiotherapy were excluded from present study.
Study Procedure
Following routine Haematoxylin and Eosin (H&E) staining, Immunohistochemical (IHC) staining was performed for Oestrogen Receptor (ER), Progesterone Receptor (PR), HER2/neu (HER2), and PD-L1 expression in all cases. The following clinicopathological parameters were studied in all cases of MBC and FBC: age of the patient, location of the mass, laterality, gross tumour size, histological type, Modified Bloom Richardson (MBR) grade, lymph node status, lymphovascular invasion, and perineural invasion.
Based on the IHC findings, all breast carcinoma cases were classified into four molecular subtypes: luminal A, luminal B, HER2 enriched, and triple negative, according to the new molecular classification. PD-L1 expression was also studied in both males and females and correlated according to molecular subtypes and clinicopathological parameters. Sections of four-micron thickness were prepared from the FFPE tissue blocks and stained with antibodies against ER (Quartett, Lot no: 4141140730), PR (Quartett, Lot no: 414907), HER2 (GenomeMe, Lot no: 030800475), and PD-L1 (Biomarq, Lot no: MM13209072021). Tonsil tissue was used as a positive control, while negative control was achieved by omitting the primary antibody in PD-L1 staining. The prognostic significance of PD-L1 was evaluated by comparing its expression with tumour grades, molecular subtypes, tumour stage, and overall survival analysis.
The patients were followed-up for a period of two years, during which overall survival was measured.
Interpretation of Immunostaining:
ER/PR: All cases with atleast 1% of positive cells were considered positive, and quantification was done using the Allred scoring system [12].
The Allred score combines the percentage of positive cells and the intensity of the reaction product in most carcinomas. The two scores are added together to yield a final score with eight possible values. Scores of 0 and 2 are considered negative, while scores of 3 to 8 are considered positive.
HER2: The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines were used for the interpretation of HER2/neu scoring [13].
For HER2 interpretation, a low-power objective (100X) was used, and immunostaining was observed in a homogeneous, contiguous population of tumour cells. No staining or membrane staining in less than 10% of tumour cells was scored as 0; faintly perceptible partial membrane staining in more than 10% of tumour cells was scored as 1+; weak or moderate complete membrane staining in more than 10% of tumour cells was scored as 2+; and strong complete membrane staining in more than 30% of tumour cells was scored as 3+. A case of gastric adenocarcinoma known to be HER2 positive served as the positive control, while the negative control was achieved by omitting the primary antibody. For the purpose of present study, scores of 2+ and 3+ were considered positive, while scores of 0 and 1+ were considered negative.
PD-L1: The interpretation of specimens was performed by a pathologist using a light microscope with an objective magnification of 10-40x. All viable tumour cells on the entire slide were evaluated and included in the PD-L1 scoring assessment. A minimum of 100 viable tumour cells must be present for the specimen to be considered adequate for PD-L1 evaluation. Any perceptible membrane staining (partial or complete) was included in the scoring. Cytoplasmic staining was considered non specific and not included as positive. Tumour-associated immune cells, such as infiltrating lymphocytes or macrophages showing cytoplasmic or membrane staining, were also included in the scoring system [14].
The specimens were considered PD-L1 positive if the Combined Positive Score (CPS)-which is the percentage of viable tumour cells showing partial or complete membrane staining, along with immune cells (lymphocytes and macrophages) exhibiting membrane or cytoplasmic staining-was ≥10% of the viable tumour cells exhibiting membrane staining at any intensity (i.e., ≥1+) [13]. Conversely, the specimens were deemed PD-L1 negative if, the CPS was <10% of the viable tumour cells exhibiting membrane staining at any intensity (i.e., ≥1+) [15].
Statistical Analysis
The SPSS version 29.0, released in 2022 by International Business Machine (IBM), was used for data analysis. A descriptive study design was employed to assess demographic and clinical features. Results were presented as numbers/percentages for categorical variables and as mean±Standard Deviation (SD)/percentages for continuous variables. The Chi-square test was utilised for correlation studies, with p-values calculated (p-values <0.05 were considered statistically significant). Kaplan’s-Meier plots were used for survival data analysis and were compared across overall cases, as well as PD-L1 positive and negative cases, in both genders.
Results
A total of 204 cases undergoing mastectomy were included in the study; 50 of them were male and 154 were female. The age range was 48 to 65 years in the male population and 30 to 74 years in the female population. The mean age±SD of the male patients was 60.72±4.458 years, which was significantly greater than the mean age of female patients (50.19±8.296).
Clinicopathological profile: The main presenting complaints of the majority of patients were a mass and pain in the breast. No significant differences were noted in the laterality of the tumour; in MBC cases, 23 (46%) cases had a right-sided lump, whereas in FBC cases, 77 (50%) cases had a right-sided breast lump. The upper outer quadrant of the breast was more commonly involved, with 29 (58%) cases in males and 93 (60.3%) cases in females [Table/Fig-1].
Clinical data of all Male Breast Carcinoma (MBC) and Female Breast Carcinoma (FBC) cases (N=204).
| Parameters | Male Breast Carcinoma (MBC) (n=50) | Female Breast Carcinoma (FBC) (n=154) |
|---|
| Age | Mean±SD (years) | 60.72±4.458 | 50.19±8.296 |
| Range (years) | 48-65 | 30-74 |
| Location of mass | Upper Outer Quadrant (UOQ) | 29 (58%) | 93 (60.3%) |
| Upper Inner Quadrant (UIQ) | 2 (4%) | 7 (4.6%) |
| Lower Outer Quadrant (LOQ) | 5 (10%) | 25 (16.2%) |
| Lower Inner Quadrant (LIQ) | 7 (14%) | 13 (8.4%) |
| UOQ+UIQ | 3 (6%) | 6 (3.9%) |
| LOQ+LIQ | 0 | 7 (4.6%) |
| LOQ+UOQ | 0 | 3 (2%) |
| Nipple areolar area | 4 (8%) | 0 |
| Laterality | Right | 23 (46%) | 77 |
| Left | 27 (54%) | 77 |
The size of tumours ranged from 1.5 to 14 cm in males and from 1.5 to 15 cm in females. Males had smaller tumours compared to females, with a mean size of 4.8±2.169 cm in males and 5.702±2.585 cm in females. The majority of tumours were in the T2 stage for both males and females 36 (72%) cases in males vs. 79 (51.3%) cases in females. In male patients, there were 46 cases (92%) of Invasive Breast Carcinoma (IBC), no special type, followed by four cases (8%) of lobular carcinoma. In females, the majority of carcinomas were also IBC, specifically ductal type 138 (89.6%) cases. The Modified Bloom Richardson (MBR) grading system was not utilised for metaplastic and mucinous breast carcinomas. Grade 2 breast tumours were the most prevalent in both males and females 38 (76%) cases in males vs. 99 (64.3%) cases in females. Grade 3 tumours were the second most common 9 (18%) cases in males vs. 36 (23.3%) cases in females. Lymph nodal metastasis was commonly associated with female breast cancer patients 35 (70%) cases in males vs. 121 (79%) cases in females). Lymphovascular invasion was frequently observed 33 (66%) cases in males vs. 109 (71%) cases in females, but perineural invasion of tumour cells was rare 8 (16%) cases in males vs. 38 (24.7%) cases in females.
Hormonal receptor status was assessed for each case, classifying them into Luminal A, Luminal B, HER2/neu enriched, and triple-negative molecular subtypes. The Luminal A subtype was most prevalent in the male population 19 (38%) cases, followed by the triple-negative subtype 15 (30%) cases. In females, the triple-negative subtype was more common 54 (35%) cases, followed by the HER2/neu enriched subtype 43 (28%) cases [Table/Fig-2].
Gross and histopathology of breast carcinoma cases.
| Parameters | | Male Breast Carcinoma (MBC) (n=50) | Female Breast Carcinoma (FBC) (n=154) |
|---|
| Tumour size | Mean±SD (cm) | 4.8±2.169 | 5.702±2.585 |
| Range (cm) | 1.5-14 | 1.5-15 |
Microscopy
Histological types
| Invasive Breast Carcinoma (IBC), NST (ductal) | 46 (92%) | 138 (89.6%) |
| Lobular carcinoma | 4 (8%) | 4 (2.6%) |
| Mucinous carcinoma | 0 | 6 (3.9%) |
| Metaplastic carcinoma | 0 | 6 (3.9%) |
Modified Bloom Richardson grade
| 1 | 3 (6%) | 7 (4.6%) |
| 2 | 38 (76%) | 99 (64.3%) |
| 3 | 9 (18%) | 36 (23.3%) |
| NA | 0 | 12 (7.8%) |
Lymph node status
| Involved | 35 (70%) | 121 (79%) |
| Uninvolved | 15 (30%) | 33 (21%) |
Molecular subtypes
| Luminal A | 19 (38%) | 33 (21%) |
| Luminal B | 7 (14%) | 24 (16%) |
| HER2/neu enriched | 9 (18%) | 43 (28%) |
| Triple negative | 15 (30%) | 54 (35%) |
Lymphovascular invasion
| Present | 33 (66%) | 109 (71%) |
| Absent | 17 (34%) | 45 (29%) |
Perineural invasion
| Present | 8 (16%) | 38 (24.7%) |
| Absent | 42 (84%) | 116 (75.3%) |
PD-L1 expression profile: A total of 24 MBC cases showed positive PD-L1 expression (48%), and in females, 81 (52.6%) cases exhibited PD-L1 positivity [Table/Fig-3]. The relationship between clinicopathological factors and PD-L1 expression is summarised in [Table/Fig-4]. In males, no significant correlation was found between PD-L1 expression and lymph nodal status, MBR grade, or lymphovascular and perineural invasion. However, a statistically significant relationship (p-value=0.011) was observed between PD-L1 status and tumour size; approximately 13 (81%) cases of T3 tumours were positive for PD-L1. Molecular subtypes also showed a statistically significant correlation with PD-L1 expression (p-value <0.001), with triple-negative tumours exhibiting a higher positivity rate for PD-L1 (86.7%) [Table/Fig-5a-f,6a-d].
Expression of PD-L1 in breast carcinomas of both gender (N=204).
| PD-L1 status | Male Breast Carcinoma (MBC) | Female Breast Carcinoma (FBC) | p-value |
|---|
| n (%) | n (%) |
| Positive | 24 (48) | 81 (52.6) | 0.572 |
| Negative | 26 (52) | 73 (47.4) |
| Total | 50 (100) | 154 (100) |
Comparison between PD-L1 status and clinicopathological profiles of both FBC and MBC.
| Parameters | Male Breast Carcinoma (MBC) | Female Breast Carcinoma (FBC) |
|---|
| PD-L1 positive | PD-L1 negative | p-value | PD-L1 positive | PD-L1 negative | p-value |
|---|
| Tumour size | 0-2 cm | 0 | 1 | 0.011 | 3 | 0 | 0.021 |
| >2-5 cm | 11 | 22 | 34 | 45 |
| >5 cm | 13 | 3 | 44 | 28 |
| Molecular subtype | Luminal A | 1 | 18 | <0.001 | 3 | 30 | <0.001 |
| Luminal B | 5 | 2 | 12 | 12 |
| HER2/neu enriched | 5 | 4 | 18 | 25 |
| Triple negative | 13 | 2 | 48 | 6 |
| Lymph node status | Involved | 18 | 17 | 0.459 | 63 | 58 | 0.80 |
| Uninvolved | 6 | 9 | 18 | 15 |
| MBRgrade | 1 | 1 | 2 | 0.432 | 4 | 3 | 0.022 |
| 2 | 17 | 21 | 45 | 54 |
| 3 | 6 | 3 | 26 | 10 |
| NA | 0 | 0 | 6 | 6 |
| LVI | Present | 17 | 16 | 0.488 | 63 | 46 | 0.044 |
| Absent | 7 | 10 | 18 | 27 |
| PNI | Present | 5 | 3 | 0.37 | 26 | 12 | 0.024 |
| Absent | 19 | 23 | 55 | 61 |
LVI: Lymphovascular invasion; PNI: Perineural invasion
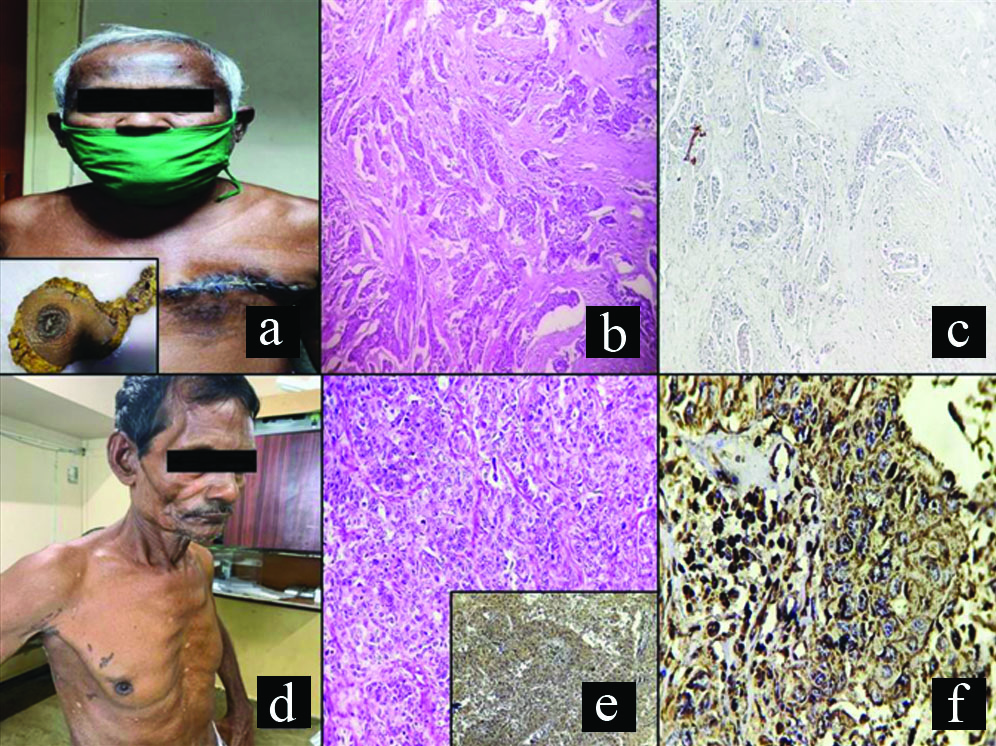
(a) Photograph of an elderly MBC patient with postoperative suture mark in left breast; inset shows gross photograph of left breast specimen with attached axillary tail; (b) Low magnification view of histology of the specimen showing an IBC NST, histological grade 2 (100X, H&E); (c) Low magnification of same specimen showing PD-L1 negativity (100X); (d) Photograph of another elderly patient with right-sided breast mass; (e) Low magnification view of histology of the specimen showing an IBC, NST, histological grade 3 (100X, H&E); inset shows low power view of strong PD-L1 membranous positive tumour cells (100X); (f) High magnification view of same showing strong membranous PD-L1 positivity (400X).
IBS NST; Invasive breast cancer of no special type
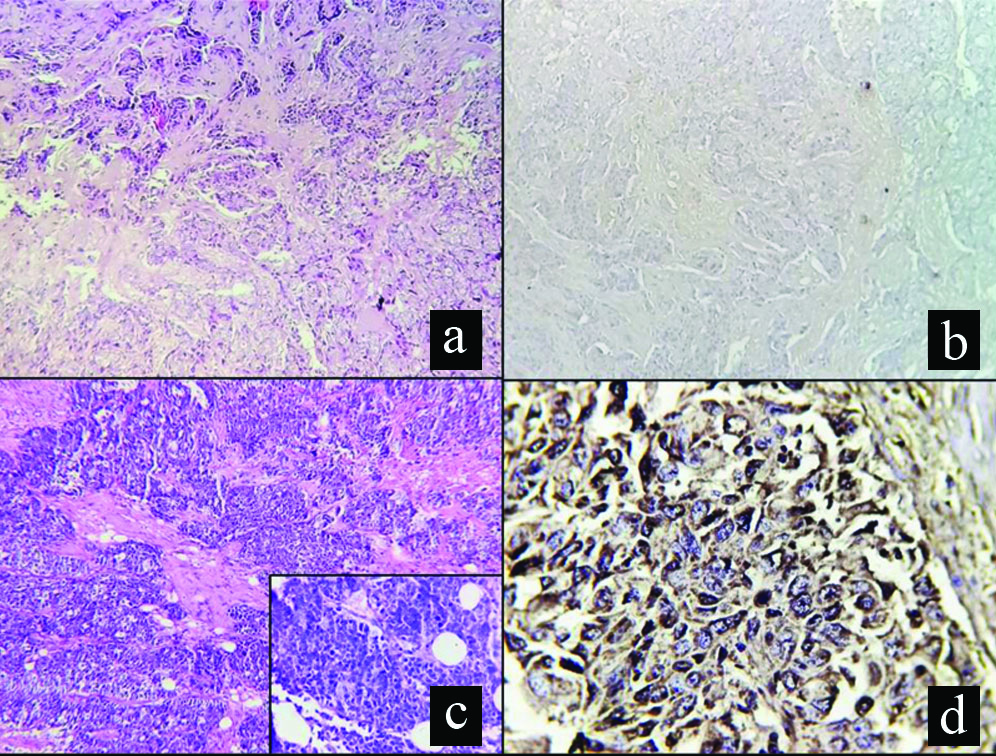
(a) Low magnification view of a female breast showing an IBC NST, histological grade 2 (100X, H&E); (b) Low magnification of same specimen showing PD-L1 negativity (100X); (c) Low magnification view of histology of another female breast specimen showing an IBC, NST, histological grade 3 (100X, H&E); inset shows high power view of the same (400X, H&E); (d) High magnification view of same showing strong membranous PD-L1 positivity (400X).
In females, a statistically significant correlation was observed between PD-L1 expression and tumour size, molecular subtypes, histological grade, and lymphovascular invasion. No significant correlation was found concerning lymph nodal status or the age of the patients. The expression of PD-L1 in the male and female populations was not drastically different; however, in males, PD-L1 expression was inconsistent and did not show significant correlation with tumour grades, which was evident in females.
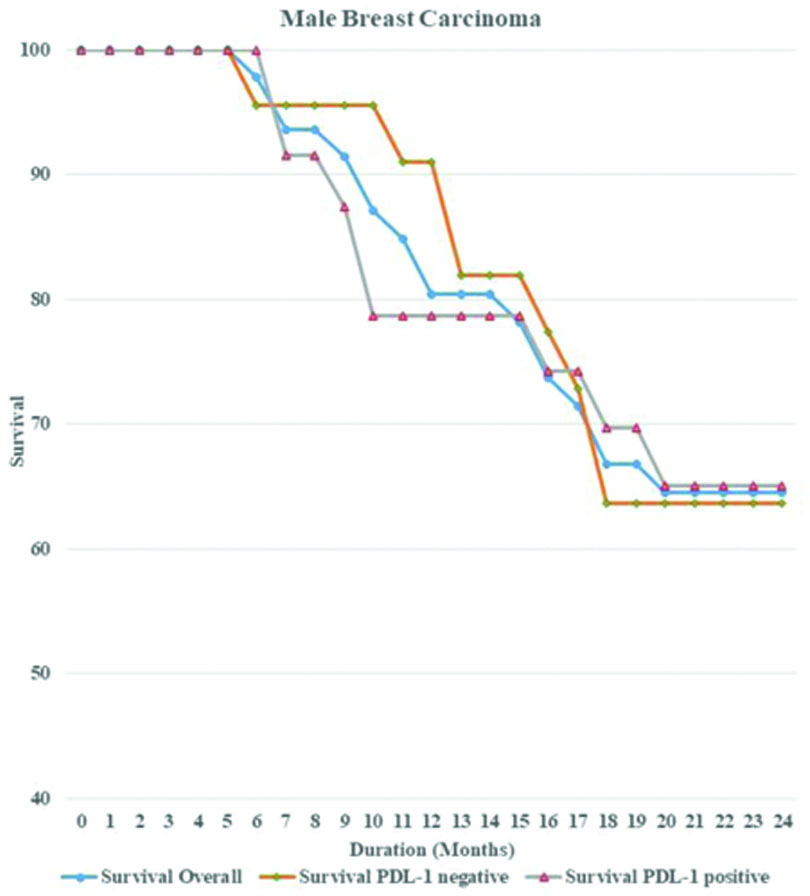
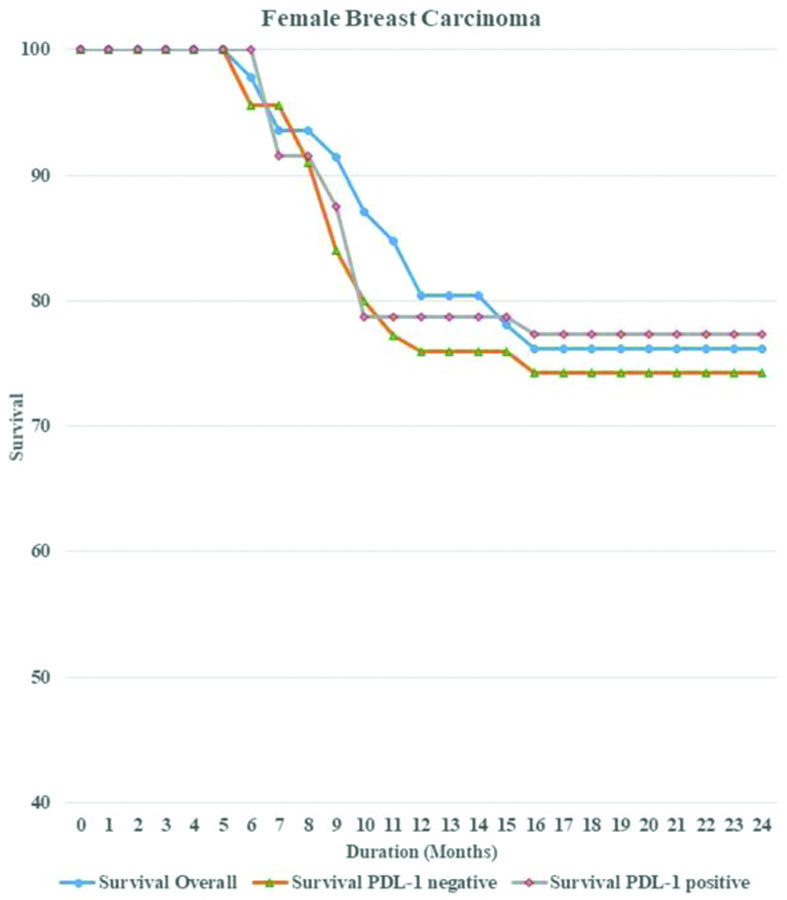
Survival analysis: Survival analysis was conducted on all patients over a 24-month period. Overall survival was measured for both PD-L1 positive and PD-L1 negative cases, separately for males and females [Table/Fig-7]. The statistical significance was determined using the Log-rank test. There was no statistically significant survival advantage associated with PD-L1 expression in either gender, as the Kaplan’s-Meier plots revealed similar results (p-value=0.85 for males and 0.78 for females). Overall survival rates were higher in females than in males, which may indicate a more adverse progression of disease in males [Table/Fig-8,9].
Survival data of both FBC and MBC after follow-up of 24 months.
| Parameters | Male Breast Carcinoma (MBC) | Female Breast Carcinoma (FBC) |
|---|
| PD-L1 positive | PD-L1 negative | p-value | PD-L1 positive | PD-L1 negative | p-value |
|---|
| Patient status after 24-month follow-up | Alive | 11 | 14 | 0.491 | 53 | 36 | 0.128 |
| Dead | 11 | 8 | 20 | 27 |
| Lost to follow-up | 2 | 4 | 8 | 10 |
Kaplan’s-Meier curve showing overall survival rate along with survival for PD-L1 positive and negative cases in MBC patients over a period of 24 months.
Kaplan’s-Meier curve showing overall survival rate along with survival for PD-L1 positive and negative cases in Female Breast Cancer (FBC) patients over a period of 24 months.
Discussion
The expression of PD-L1 in FBC is a well-studied fact, and several immune checkpoint inhibitors directed against PD-1/PD-L1 have been included in treatment protocols. The main aim of present study was to evaluate PD-L1 expression in MBC cases and to compare it with FBC.
The study results indicated that the average age of presentation for MBC (60.72 years) was higher than that for FBC (50.19 years). Similar results have been observed in many studies [16,17]. A study by Massarweh SA et al., showed that the average age of presentation was 64.2 years in the selected cohort [18]. The size of tumours was smaller in males than in females, with an average breast tumour size of 4.8 cm in males compared to 5.7 cm in females. This result is supported by observations reported by Manson QF et al., [10].
The majority of MBC cases were classified as MBR grade 2 (76%), and 46 (92%) out of 50 MBC cases showed IBC, NST histology. The remaining 4 (8%) cases exhibited lobular carcinoma histology. In contrast, FBC had 138 cases (89.6%) of IBC, NST out of 154 cases, while 4 cases (2.6%) had lobular morphology, and 6 cases (3.9%) each had mucinous and metaplastic histology. In the MBR grade group, 99 cases (64.3%) were classified as MBR grade 2, whereas 36 cases (23.4%) had MBR grade 3, which was higher compared to MBC (18% in MBC).
Molecular subgrouping of breast cancers revealed the luminal subtype to be the most prevalent in MBC cases (26 cases, 52%). Triple-negative cases constituted 15 cases (30%), and HER2/neu-enriched cases accounted for 9 (18%). Molecular analysis of FBC showed that a major portion of cases were either triple-negative 54 (35%) cases or HER2/neu-enriched 43 (27.9%) cases.
The clinicopathological status of present study demonstrated that MBCs were predominantly of MBR grade 2, with IBC, NST histology and a luminal-type molecular (IHC) profile. Previous studies on breast carcinoma have shown similar results. One study by Brcic I et al., also indicated that the HER2/neu variant was significantly lower in the study cohort of MBC; however, that was not the case in the present study [11]. Several other studies have reported HER2/neu expression in MBC ranging between 2 to 27% [19,20].
The study demonstrated only slightly reduced PD-L1 expression in MBC compared to FBC. This slight difference can be explained by the increased number of luminal molecular types (ER/PR positive) in MBC compared to FBC; according to the literature, PD-L1 expression is strongly associated with TNBC [8]. This observation was not statistically significant (p-value=0.515).
PD-L1 expression in both genders was also compared with tumour size, molecular subtype, MBR grade, lymph nodal status, and lymphovascular and perineural invasion. In this clinicopathological feature-based analysis, PD-L1 expression was associated with tumour size and molecular subtypes in both genders. The correlations were statistically significant (Tumour size: p-value=0.011 in males and 0.021 in females; molecular subtype: p-values <0.001 in both genders). No significant correlations were found with histological grades, lymphovascular invasion, or perineural invasion in males.
However, PD-L1 expression was associated with higher histological grade (p-value=0.022), lymphovascular invasion (p-value=0.044), and perineural invasion (p-value=0.024) in females.
The PD-L1 expression status in MBC and FBC did not support previous studies, as described by Manson QF et al., and Brcic I et al., which found significantly reduced PD-L1 expression in MBC compared to FBC [10,11]. In the study by Manson QF et al., PD-1 expression on tumour-infiltrating lymphocytes was significantly less frequent in male cancers than in female cancers (48.9% vs. 65.3%) [10]. In contrast, PD-L1 expression on tumour and immune cells did not differ significantly [14]. The relationship between different clinicopathological parameters yielded mixed results in the literature [21-25]. In a study by Ghebeh H et al., PD-L1 expression was significantly associated with higher histological grade (p-value=0.036), ER and PR negativity, and PD-L1 expression in tumour-infiltrating lymphocytes was associated with larger tumour size (p=0.042), higher histological grade (p=0.015), and positive HER2/neu status (p=0.019) [21]. No significant relationship with age, lymph nodal status, or treatment with neoadjuvant chemotherapy was established [21]. In another study by Guo L et al., PD-L1 expression was associated with a slightly younger age group (p=0.09) and higher tumour grade (p=<0.0001). No significant differences were observed regarding lymph nodal status, histologic subtype, tumour size, or basal-like subtype [25].
In the overall survival analysis, the significant prognostic value of PD-L1 was not observed in tumours in either gender. The literature also shows that the prognostic value of PD-1/PD-L1 in FBC is equivocal, with many studies revealing no significant prognostic values [25,26].
Limitation(s)
The study conducted was a single-centre, tertiary care study, which may not be representative of the entire community. The scope of present study is limited due to the small sample size and the restricted time period. Additionally, the study did not utilise fluorescent in-situ hybridisation or polymerase chain reaction techniques for the analysis of PD-L1 expression.
Conclusion(s)
The expression of PD-L1 in Metastatic Breast Cancer (MBC) does not differ significantly from that in Female Breast Cancer (FBC), although intrinsic pathogenesis differences are evident between MBC and FBC. There was no significant statistical relationship between PD-L1 expression and survival analysis for both genders; however, the overall survival rate was better in females than in males.
LVI: Lymphovascular invasion; PNI: Perineural invasion