Breast cancer is a heterogeneous disease with varying molecular subtypes and poses a significant health challenge worldwide. It remains the most commonly diagnosed cancer among women, and its complexity is underscored by the diverse clinical outcomes and therapeutic responses observed across patients. In the quest to better understand the molecular underpinnings of breast cancer, researchers have identified a plethora of biomarkers and signaling pathways that play crucial roles in its development, progression, and response to treatment. With high-throughput technologies, particularly microarray analysis, a new taxonomy for breast cancer based on molecular features has been proposed. At least five molecular breast cancer subtypes have been described: luminal A, luminal B, HER2-enriched, basal-like, and normal breast-like [1]. Female breast cancer was the second leading cause of global cancer incidence in 2022, with an estimated 2.3 million new cases, comprising 11.6% of all cancer cases. The disease is the fourth leading cause of cancer mortality worldwide, with 666,000 deaths (6.9% of all cancer deaths). Among women, breast cancer is the most commonly diagnosed cancer and is the leading cause of cancer deaths globally. According to the International Agency for Research on Cancer (IARC), one in 12 women will be diagnosed with breast cancer in their lifetime, and one in 71 women will die from it in countries with a very high Human Development Index (HDI). In contrast, in countries with a low HDI, while only one in 27 women is diagnosed with breast cancer in their lifetime, one in 48 women will die from it [2].
GATA3 is a member of the GATA family of zinc-finger binding transcription factors that regulate the specification and differentiation of many tissue types, including the breast, kidney, T cells, nervous system, and hair follicles. Although GATA3 is expressed in a wide variety of tissues, the expression of transcription factors in many tissues is at low levels that are not detectable by Immunohistochemistry (IHC). Immunohistochemical labeling for GATA3 in normal tissues is far more restricted, and GATA3 labeling has been demonstrated to be a highly specific marker for breast carcinomas and urothelial carcinomas [3]. In the mammary gland, GATA3 facilitates the differentiation of luminal epithelial cells. The knockout of GATA3 at different stages of development leads to a failure in luminal progenitor specification and inhibits differentiation [4]. The relationship between GATA3 and breast cancer has prompted its exploration as a diagnostic and prognostic marker. Additionally, predictive and prognostic factors, such as the expression of ER and PR, indicate that genes like GATA3 are involved in similar pathways and have clinical relevance. The GATA3 gene encodes the GATA-3 protein, which is located on chromosome 10 and is involved in the development and differentiation of normal breast tissue, acting as a transcription factor [5]. Several studies using animal models, cell lines, and clinical samples have demonstrated that the loss of GATA3 expression is correlated with an aggressive phenotype of breast cancer [6]. GATA3 expression has been examined in various breast cancer subtypes; however, its correlation with clinicopathological features remains unclear. Therefore, whether GATA3 serves as a predictive factor for disease-free survival or as a prognostic marker is inconclusive [7]. GATA3-positive tumours are crucial and are likely derived from luminal progenitors, which help maintain the luminal epithelial cell phenotype. GATA3-positive tumours are often ER-positive, and this association significantly indicates that GATA3 expression is linked to a favourable prognosis. Therefore, the clinicopathological characteristics of GATA3-positive tumours and the relationship between GATA3 expression and response to neoadjuvant chemotherapy for breast cancer are noteworthy [5].
This study aimed to evaluate the relationship between GATA3 expression and clinicopathological factors, as well as the molecular subtypes of breast cancer. Thus, GATA3 emerges as a multifaceted player in the intricate landscape of breast cancer biology. The clinical significance of GATA3 expression by examining its association with clinicopathological parameters, including age, tumour size, tumour type, histologic grade, lymph node involvement, and NPI status, as well as with molecular subtype using a panel of breast cancer immunohistochemical markers such as ER, PR, and HER2/Neu was done.
Materials and Methods
This cross-sectional study was conducted at Pt. BD Sharma, PGIMS, Rohtak, Haryana, India, after obtaining approval from the Biomedical Research Ethics Committee (certificate number: EC/NEW/INST/2022/HR/0189). The study involved 60 cases of breast carcinoma and was completed over a one-year period from April 2023 to March 2024.
Inclusion criteria: The study material consisted of modified radical mastectomy specimens submitted to the Department of Pathology.
Exclusion criteria: Trucut and excisional biopsies, as well as breast malignancies other than carcinoma, were excluded from the study.
Morphological evaluation: Histopathological diagnoses were established using routine Haematoxylin and Eosin (H&E) stained sections. Specimens were examined for tumour size, tumour grade, NPI, and lymph node status. Histological tumour grading was performed using the Nottingham MBR grading system [8,9].
Immunohistochemical analysis: Paraffin sections (3-4 μm) were subjected to IHC for ER, PR, Her2/Neu, Ki67, and GATA3. The sections for GATA3 were deparaffinised and incubated with anti-GATA3 antibodies (DAKO), followed by incubation with a secondary antibody for one hour at room temperature. Subsequent treatment with DAB (3,3-Diaminobenzidine-tetrahydrochloride) solution for 10 minutes facilitated visualisation. Positive and negative controls were used for each run.
The expression of ER and PR was assessed using Allred scoring, while HER2/neu was evaluated using the HER2/neu scoring system [10,11]. The scoring method for GATA-3 expression was based on a semiquantitative scoring system [5]. In this system, the percentage of staining was categorised as follows: 0 = no nuclear expression; 1=1 to 10% positive tumour nuclei; 2=11 to 20%; and so on, up to a maximum score of 10=91 to 100% positive tumour nuclei. The intensity was scored as follows:
1+ = weak staining;
2+ = moderate staining;
3+ = strong staining.
The final numeric score was generated by multiplying the percentage by the intensity of nuclear expression (scoring = percentage×intensity). Based on this semiquantitative scoring system, scores between zero and three were classified as negative, while scores of four or higher, up to a maximum of 30, were considered positive. Ductal epithelial cells in benign lobules served as positive controls, and negative controls were obtained by substituting the primary antibody with an antibody of non specific relevance.
Statistical Analysis
The collected data were analysed with the help of a software package (SPSS version 24.0). All the data listed in the investigation proforma (name, age, sex, CR number, clinical diagnosis, and history) were collected. The association was tested using the Chi-square test and Fisher’s exact Test. A p-value <0.05 was accepted as statistically significant.
Results
The present study was a descriptive investigation conducted on 60 cases of breast carcinoma. The ages of the participants ranged from 28 to 75 years, with a median age of 50.00 years (interquartile range: 40.5-58.5). The maximum number of cases (33 cases; 55%) were in the age group of 41-60 years. The tumours were equally distributed on both sides, with 50% on the left and 50% on the right. According to tumour size, the majority of the cases, i.e., 44 (73.33%), belonged to the subgroup measuring 2-5 cm. Invasive Ductal Carcinoma-Not Otherwise Specified (IDC-NOS) was the most common histologic subtype, accounting for 56 cases (93.33%). Two cases (3.33%) were identified as IDC with medullary features, while other histologic subtypes included one case (1.67%) each of lobular and mucinous carcinoma. Thirty-eight cases (63.33%) were classified as Grade II (moderately differentiated). Lymph nodes were involved in 32 cases (53.33%). The majority of the cases, i.e., 39 (65%), fell into the moderate prognostic group. In terms of IHC, molecular subtyping was performed. GATA-3 expression in this study demonstrated a sensitivity of 75% in primary breast carcinomas, which was significantly high [Table/Fig-1].
Clinicopathological parameters of the study (n=60).
| Parameters | Frequency (%) |
|---|
| Age (years) |
| 21-30 | 4 (6.67) |
| 31-40 | 11 (18.33) |
| 41-50 | 19 (31.67) |
| 51-60 | 14 (23.33) |
| 61-70 | 8 (13.33) |
| 71-80 | 4 (6.67) |
| Side |
| Right | 30 (50) |
| Left | 30 (50) |
| Tumour size (cm) |
| <2 | 7 (11.67) |
| 2-5 | 44 (73.33) |
| >5 | 9 (15.0) |
| Histological subtype |
| IDC-NOS | 56 (93.33) |
| IDC with medullary features | 2 (3.33) |
| Invasive lobular carcinoma | 1 (1.67) |
| Invasive mucinous carcinoma | 1 (1.67) |
| Histological grade |
| I | 12 (20) |
| II | 38 (63.33) |
| III | 10 (16.67) |
| Lymph node involvement |
| 0 node | 28 (46.67) |
| 1-3 nodes | 14 (23.33) |
| 4-9 nodes | 14 (23.33) |
| ≥10 nodes | 4 (6.67) |
| NPI category |
| Good | 11 (18.33) |
| Moderate | 39 (65) |
| Poor | 10 (6.67) |
| ER |
| Positive | 27 (45.0) |
| Negative | 33 (55.0) |
| PR |
| Positive | 21 (35) |
| Negative | 39 (65) |
| HER2/NEU |
| Positive | 15 (25) |
| Negative | 45 (75) |
| Molecular subtype |
| Luminal A | 16 (26.67) |
| Luminal B | 12 (20) |
| HER2 enriched | 12 (20) |
| Triple negative/Basal Like | 20 (33.33) |
| GATA3 |
| Positive | 45 (75) |
| Negative | 15 (25) |
ER: Oestrogen receptor; PR: Progesterone receptor; NPI: Nottingham prognostic index; IDC-NOS: Invasive ductal carcinoma-not otherwise specified
The primary objective of this study was to evaluate the significance of GATA-3 immunohistochemistry in primary breast carcinoma, including triple-negative breast carcinomas, and its association with various clinicopathological parameters such as age, tumour side, tumour size, histologic type, molecular subtype, histologic grade, lymph node status, and NPI status of the tumour wherever possible. No association was observed between GATA-3 expression and the age of the patient, side of the breast involved, tumour size, histologic subtype, lymph node status, NPI, or HER2/Neu status of the tumour. However, GATA-3 expression showed a direct association with histological grade (p-value=0.024) and ER expression (p-value <0.001) and PR expression (p-value=0.001) [Table/Fig-2].
Association between GATA-3 expression, clinicopathological parameters and molecular subtypes.
| Parameters | GATA3, n (%) | p-value |
|---|
| Positive (n=45) | Negative (n=15) |
|---|
| Age (years) |
| 21-30 | 4 (8.88) | 0 | 0.430 |
| 31-40 | 6 (13.33) | 5 (33.33) |
| 41-50 | 15 (33.33) | 4 (26.66) |
| 51-60 | 10 (22.22) | 4 (26.66) |
| 61-70 | 7 (15.55) | 1 (6.66) |
| 71-80 | 3 (6.66) | 1 (6.66) |
| Side |
| Right | 23 (51.11) | 7 (46.67) | 0.412 |
| Left | 22 (48.89) | 8 (53.33) |
| Tumour size (cm) |
| <2 | 7 (15.56) | 0 | 0.088 |
| 2-5 | 34 (75.56) | 10 (66.67) |
| >5 | 4 (8.88) | 5 (33.33) |
| Histological subtype |
| IDC-NOS | 41 (91.11) | 15 (100.0) | 1.000 |
| IDC with medullary features | 2 (4.44) | 0 |
| Invasive lobular carcinoma | 1 (2.22) | 0 |
| Invasive mucinous carcinoma | 1 (2.22) | 0 |
| Histological grade |
| I | 11 (24.45) | 1 (6.67) | 0.024 |
| II | 28 (62.22) | 10 (66.67) |
| III | 6 (13.33) | 4 (26.66) |
| LN involvement |
| 0 node | 22 (48.88) | 6 (40.0) | 0.317 |
| 1-3 nodes | 9 (20.0) | 5 (33.33) |
| 4-9 nodes | 10 (22.22) | 4 (26.67) |
| ≥10 nodes | 4 (8.88) | 0 |
| NPI category |
| Good | 10 (22.22) | 1 (6.66) | 0.239 |
| Moderate | 29 (64.44) | 10 (66.67) |
| Poor | 6 (13.33) | 4 (26.66) |
| ER |
| Positive | 27 (60.0) | 0 | <0.001 |
| Negative | 18 (40.0) | 15 (100) |
| PR |
| Positive | 21 (46.66) | 0 | 0.001 |
| Negative | 24 (53.33) | 15 (100.0) |
| HER2/NEU |
| Positive | 12 (26.67) | 3 (20.0) | 0.738 |
| Negative | 33 (73.33) | 12 (80.0) |
| Molecular subtype |
| Luminal A | 16 (35.56) | 0 | <0.001 |
| Luminal B | 12 (26.66) | 0 |
| HER2 enriched | 9 (20.0) | 3 (20.0) |
| Triple negative/Basal like | 8 (17.78) | 12 (80.0) |
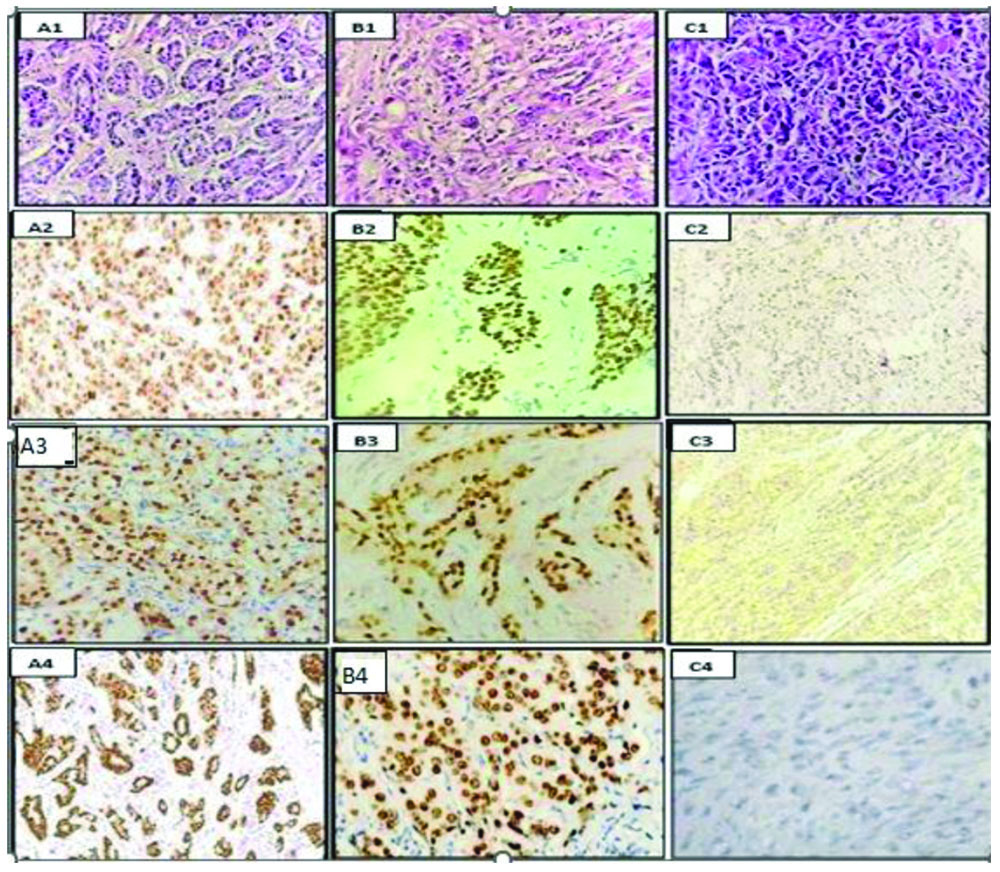
When comparing the expression of GATA-3 with molecular subtypes, all GATA-3 positive cases (100%) were classified within the luminal A and luminal B subtypes (p-value <0.001). In contrast, triple-negative subtypes were negative for GATA-3 expression [Table/Fig-3].
Luminal A molecular subtype: (A1) Infiltrating Ductal Carcinoma- NOS, Nottingham Grade-I, (H&E stain, 400x); (A2) Nuclear positivity for ER, (IHC stain, 400x);(A3) Nuclear positivity for PR, (IHC stain, 400x); (A4) Nuclear positivity for GATA3, (IHC stain, 100x).
Luminal B molecular subtype: (B1) Infiltrating Ductal Carcinoma, Nottingham Grade II, (H&E stain, 400x); (B2) Nuclear positivity for ER, (IHC stain, 400x); (B3) Nuclear positivity for PR, (IHC stain, 400x); (B4) Nuclear positivity for GATA3, (IHC stain, 400x).
Triple negative/Basal like molecular subtype: (C1) Infiltrating Ductal Carcinoma, Nottingham Grade III, (H&E stain, 400x); (C2) Negative ER expression, (IHC stain, 100x); (C3) Negative PR expression, (IHC stain, 400x); (C4) Negative expression of GATA3, (IHC stain, 400x).
Discussion
GATA3 is a transcription factor that plays a pivotal role in the development and maintenance of the breast tissue. In breast cancer, its significance lies in its dual nature- acting as a tumour suppressor or an oncogene depending on the context. Aberrant GATA3 expression has been observed in various breast cancer subtypes, highlighting its potential as a diagnostic and prognostic marker [12]. A lack of GATA3 expression leads to chemotherapy resistance and a mesenchymal phenotype in cancer. Functionally, GATA3 expression in breast cancer cells reduces tumour-initiating ability, epithelial-to-mesenchymal transition, and metastatic potential [13].
GATA3 expression was assessed based on a semiquantitative scoring system, where scores between zero and three were labeled as negative, and scores of four or higher, up to a maximum of 30, were considered positive. Forty-five cases (75%) showed positivity for GATA3. These results are consistent with various studies in the literature that documented GATA3 sensitivities ranging from 32 to 92%. Specifically, Cimino-Mathews A et al., reported 67%, Gonzalez RS et al., reported 84%, Tominaga N et al., reported 57%, Voduc D et al., reported 32%, Albergaria A et al., reported 48%, Davis DG et al., reported 76.8%, and Rao M et al., reported 92% [3,5,12,14-17]. The variable results can be attributed to differences in sample size, the choice of antibody, and various scoring systems, including differences in the threshold for nuclear labeling of GATA3, preanalytical factors, and the number of cases with different histological subtypes.
Of the 45 cases positive for GATA3, 25 cases (55.56%) belonged to the age group of 41-60 years. There was no significant association between age and GATA3 expression. Studies conducted by Gonzalez RS et al., and Na K et al., also found no significant association between these two parameters [5,18]. However, Voduc D et al., reported a significant linear association between age and GATA3 expression [14].
Breast cancer cases with tumour sizes greater than 2 cm showed the highest positivity for GATA3. Out of the 45 cases that were positive for GATA3, 34 cases (75.56%) had tumour sizes ranging from 2 to 5 cm. There was no statistically significant association between GATA3 expression and tumour size. The results of studies conducted by Albergaria A et al., and Tominaga N et al., were similar to the findings of our study, while results from Voduc D et al., indicated a significant association with smaller tumour sizes [12,14,15].
The most common histological subtype associated with positive GATA3 expression was IDC of NOS. Due to the paucity of subtypes other than IDC-NOS cases in present study, no a statistically significant association between histological subtypes and GATA3 expression was found. Tominaga N et al., and Albergaria A et al., have shown a significant association with the histologic subtype (p-value=0.001) [12,15].
GATA3 showed higher positivity in grade II tumours (28 cases; 62.22%) compared to grade I tumours (11 cases; 24.44%), with the least positivity observed in grade III tumours (6 cases; 13.33%). The association between GATA3 expression and histological grade was statistically significant. Studies conducted by Gonzalez RS et al., Tominaga N et al., and Albergaria A et al., also demonstrated a significant association between these two parameters [Table/Fig-4] [5,12,15].
Association of GATA3 with histologic grade in other studies [5,12,15].
| Study (year) (No. of cases) | GATA3 | Grade-I | Grade-II | Grade-III | p-value |
|---|
| Gonzalez RS et al., [5] (2013) (n=163) | (+) 134 | 40 | 70 | 24 | 0.085 |
| (-) 29 | 01 | 06 | 22 |
| Tominaga N et al., [12] (2012) (n=130) | (+) 74 | 17 | 48 | 09 | <0.001 |
| (-) 56 | 01 | 38 | 17 |
| Albergaria A et al., [15] (2009) (n=204) | (+) 97 | 16 | 56 | 25 | 0.013 |
| (-) 107 | 24 | 40 | 43 |
| Present study, (2024) (n=60) | (+) 45 | 11 | 28 | 06 | 0.024 |
| (-) 15 | 01 | 10 | 04 |
In present study, the maximum number of GATA3-positive cases (22 cases; 48.8%) showed no nodal involvement. No significant association was found between GATA3 expression and nodal involvement in this study, which was consistent with findings from Gonzalez RS et al., Tominaga N et al., Albergaria A et al., and Mehra R et al., [5,12,15,19]. The lack of association may be attributable to limited follow-up of cases in these studies.
In present study, the highest number of positive GATA3 cases (n=45) was found in moderate (29 cases; 64.44%) and good (10 cases; 22.22%) prognostic groups. There was no statistically significant association between GATA3 expression and NPI category. Similarly, Albergaria A et al., observed no association between GATA3 and NPI (p-value=0.29) [15].
ER and PR expression in GATA3-positive cases showed a significant relationship. The majority of GATA3-positive cases were positive for ER and PR, which aligns with the fact that GATA3 is a regulator of luminal cell differentiation in the mammary gland. Present study results are consistent with those of Gonzalez RS et al., Tominaga N et al., and Albergaria A et al., regarding GATA3 positivity with ER and PR [5,12,15].
HER2/neu expression in GATA3-positive cases did not show a significant relationship. Most GATA3-positive cases were negative for HER2/neu (33 cases; 73.3%). Nearly all other studies corroborated to present study [5,12,18].
The maximum number of GATA3-positive cases (16 cases; 35.55%) was diagnosed as the Luminal A subtype, followed by the Luminal B subtype, which comprised 12 cases (26.67%) of positive cases. The majority of triple-negative cases (12 cases; 80%) were negative for GATA3. There was a significant association between GATA3 and molecular subtype expression, and the results were consistent with those of other studies [Table/Fig-5] [15,18,20].
Association of GATA3 with molecular subtypes in other studies [15,18,20].
| Study (year) (No. of cases) | GATA3 | Luminal A | Luminal B | HER2 enriched | Triple-negative | p-value |
|---|
| Albergaria A et al., * [15] (2009) (n=204) | (+) 97 | 78 | 03 | 02 | 06 | <0.001 |
| (-) 107 | 36 | 05 | 24 | 33 |
| Na K et al., [18] (2022) (n=84) | (+) 70 | 21 | 23 | 10 | 16 | 0.001 |
| (-) 14 | 01 | 01 | 01 | 11 |
| Kong X et al., [20] (2022) (n=228) | (+) 193 | 41 | 101 | 37 | 14 | <0.0001 |
| (-) 35 | 00 | 03 | 14 | 18 |
| Present study(2024) (n=60) | (+) 45 | 16 | 12 | 09 | 08 | <0.001 |
| (-) 15 | 00 | 00 | 03 | 12 |
*In Albergaria A et al., all the GATA3 positive and negative cases were not able to be subtyped on molecular basis
The primary objective of this study was to evaluate the significance of GATA-3 IHC in primary breast carcinoma, including triple-negative breast carcinomas, and its association with various clinicopathological parameters and molecular subtypes.
In the current study, GATA-3 expression demonstrated a sensitivity of 75% (45 out of 60 cases) in primary breast carcinomas. The higher sensitivity and staining pattern of GATA-3, along with the absence of background staining, are particularly beneficial in cases where the tissue sample is scant. Furthermore, GATA-3 exhibits nuclear expression, which facilitates better interpretation.
Present study found that GATA-3 expression was significantly correlated with hormone receptor status, molecular subtypes, and tumour grading. The results indicated that GATA-3-positive tumours exhibited a phenotype characteristic of luminal A and luminal B tumours, along with ER and PR positivity (both in terms of staining intensity and percentage of tumour cells) and tumour grading (showing a linear correlation with Grade I and II). Similar results were reported in a study conducted by Ricks-Santi LJ et al., which revealed a statistically significant association between GATA-3 expression and lower grade (p-value <0.001), ER positivity (p-value <0.001), PR positivity (p-value <0.001), and the luminal subtype (p-value <0.001), with expression predominantly found in luminal breast cancers. However, there was no association with recurrence-free or overall survival [21].
Limitation(s)
The limitations of this study included a moderate sample size with limited histological subtypes. Authors were unable to assess the relationship between GATA3 and the different histological subtypes. Additionally, many of the patients were lost to follow-up; therefore, the role of GATA3 as an independent prognostic parameter could not be established.
Conclusion(s)
Present study established a statistically significant association between GATA3 and ER and PR status, Luminal A and B molecular subtypes, and histological grade in cases of breast carcinoma. With superior sensitivity compared to Mammoglobin and GCDFP-15, GATA3 may serve as a potential marker for confirming a breast site of origin in metastatic breast carcinoma. However, the main drawback of GATA3 as a breast lineage immunomarker is its low sensitivity in the Triple Negative Breast Cancer (TNBC) molecular subtype, where a combination of markers can be used to improve detection. Tumours that express GATA3 are associated with better outcomes. Therefore, research on GATA3 expression in breast carcinoma is recommended on a larger scale, with follow-up and survival analyses to validate the role of GATA3 in the aetiology and progression of breast cancer, as well as to design prognostic groups and treatment strategies.
ER: Oestrogen receptor; PR: Progesterone receptor; NPI: Nottingham prognostic index; IDC-NOS: Invasive ductal carcinoma-not otherwise specified
*In Albergaria A et al., all the GATA3 positive and negative cases were not able to be subtyped on molecular basis