Introduction
When there is significant furcation involvement, mechanical debridement without surgery can cause the disease to progressively worsen. To halt the disease process, surgery allows for odontoplasty, root debridement, osseous recontouring, and periodontal regeneration while preserving periodontal attachment.
Need of the study
In cases of furcation deficiencies, periodontal regeneration with different bone grafts is an effective method to both replace lost bone tissue and slow down the progression of the disease. In osseous abnormalities, the additive actions and features of Cissus Quadrangularis (CQ) demonstrate improved healing. Thus, the purpose of present clinical trial is to determine whether the combination of CQ, when utilised locally and systemically, is efficacious for the management of Class II furcation defects.
Aim
To evaluate and compare the efficacy of CQ (Hadjod) used locally, systemically, and in combination as an adjunct to osteon II with Platelet Rich Fibrin (PRF) for the management of Class II furcation defects.
Materials and Methods
A randomised clinical trial will be conducted in the Department of Periodontics at Sharad Pawar Dental College, Datta Meghe Institute of Higher Education and Research (DMIHER), Wardha, Maharashtra, India from April 2024 to March 2026. A total 60 systemically healthy individuals with Class II furcation invasions, either buccal or lingual, and moderate to severe chronic periodontitis will be selected. The study population will be categorised into three interventional groups and one control group as follows: Group A: Control group (n=15) will be treated with osteon II+PRF membrane+antibiotic coverage for Class II furcation defects, Group B: (n=15) will be treated with osteon II+PRF membrane+CQ graft (locally) for Class II furcation defects+antibiotic coverage, Group C: (n=15) will be treated with osteon II+PRF membrane+systemic dosage of CQ for Class II furcation defects+antibiotic coverage, Group D: (n=15) will be treated with osteon II+PRF membrane+CQ graft (locally)+systemic dosage of CQ for Class II furcation defects+antibiotic coverage. The data will be analysed and calculated using the Student’s t-test, with a significance level of less than 0.05.
Introduction
Furcation involvement is one of the critical anatomical structures that require debridement with a basic set of periodontal instruments [1]. Due to the continuous loss of the periodontal attachment apparatus, long Class II furcations have become a significant concern for dental clinicians [2]. The goal of periodontal treatment is to remove bacterial infection through mechanical debridement [3]. Surgical access has been a treatment option for managing severe forms of furcation involvement. This surgical procedure also facilitates periodontal regeneration, helping to preserve periodontal attachment and halt disease progression [4]. The Guided Tissue Regeneration (GTR) concept advocates for the placement of a barrier (membrane) to isolate the root surface from gingival tissues. Various bone graft materials and membranes have been tested as integral components of the GTR concept for regenerative procedures [5].
In recent times, several medical and scientific fields have focused attention on Cissus quadrangularis, commonly known as Hadjod, a natural remedy. This perennial herb is native to regions of India, Sri Lanka, and Africa. Ancient Ayurvedic literature, including texts such as Bhava Prakasha and Chakra Dutta, details the use of this herb as a healing supplement for patients with fractures. Bonesetters have been using it as both an external and internal medication for centuries [6-8]. Cissus quadrangularis possesses several distinct qualities, including analgesic, anti-inflammatory, antioxidant, bone-healing, and antibacterial properties [9]. Periodontal defects are no longer an obstacle to using Cissus quadrangularis, which offers hope for periodontal tissue regeneration.
Alloplastic materials serve as scaffolds and offer several benefits, including availability, ease of manipulation, fewer complications, and high safety [10]. Among the various alloplastic materials currently available, calcium phosphate ceramics significantly aids in periodontal regeneration. Recently, various calcium phosphate ceramics have gained recognition as off-the-shelf artificial grafting materials for restoring periodontal osseous defects [11]. However, the use of Hydroxyapatite (HA) has been reported to fail in establishing new periodontal tissue attachment, osteogenesis, or cementogenesis in periodontal osseous defects [12]. The newly developed biphasic calcium phosphate ceramics {Hydroxyapatite-Beta-Tricalcium Phosphate 30/70 (HA-β-TCP 30/70)}, such as osteon II, exhibit a reticular structure that appears to enhance resorption and promote increased bone formation [13].
Among the graft materials used in maxillary sinus augmentation procedures, biphasic calcium phosphate ceramics, formed by mixing HA and Tricalcium Phosphate (TCP), are considered biocompatible, osteoconductive, and suitable for promoting bone formation [14,15]. HA, which undergoes slow resorption, functions as a scaffold to maintain space [16]. Numerous bioceramic materials are currently in use, and variations in the HA/TCP ratio, phase composition, formulation, sizes, and shapes affect their biological and mechanical properties. HA and β-TCP ceramics form a robust direct bond with the host bone [17,18].
Platelet Rich Fibrin (PRF) is a second-generation platelet concentrate [19]. PRF is a fibrin matrix that traps platelet cytokines, growth factors, and cells before releasing them after a specific period. It has the potential to be used as a resorbable membrane and is a well known alternative to more expensive membranes, demonstrating promising outcomes [20].
Considering the individual beneficial properties of osteon II bone graft and PRF, their combination could provide a novel approach with the additional osteogenic effect of CQ in Class II furcation defects. Based on the collected data, the combined local and systemic use of CQ for treating Class II furcation defects has yet to be reported. Therefore, it would be interesting to evaluate CQ’s efficacy when used both locally and systemically in Class II furcation defects. To the best of authors knowledge, this is the first clinical research to use the biomaterials.
Aim of the study and objectives: To evaluate and compare the efficacy of Cissus quadrangularis (CQ, Hadjod) used locally, systemically, and in combination as an adjunct to osteon II with Platelet-Rich Fibrin (PRF) for the management of Class II furcation defects.
Review of Literature
The CQ has shown improved osseous healing in periodontal defects with local application [21]. In their pilot trial, Managutti A et al., found that the systemic use of C. quadrangularis following implant insertion significantly impacted pain and oedema management [22]. The authors also recommended its use after dental implant placement to minimise the osteointegration period.
A randomised controlled study by Jain M et al., suggested using bovine-derived HA as a carrier to encourage the sustained release of CQ in infrabony defects for better regeneration. Bio-oss (a bovine-derived bone mineral matrix) is a well-tolerated tissue bone graft material with considerable promise as a scaffold to facilitate osseointegration [23].
However, one of the primary disadvantages of Bio-oss is the potential risk of disease transmission, along with the possibility of a reaction from the host immune system. Due to these disadvantages and the limitations of allografts and xenografts, greater attention has been focused on alloplastic materials [24].
Objectives
To evaluate the efficacy of the osteon II+PRF membrane in Class II furcation defects in terms of decreased Probing Pocket Depth (PPD), increased Clinical Attachment Level (CAL) gain, and radiographic bone fill in furcation defects.
To evaluate the efficacy of osteon II+PRF membrane+CQ used locally in Class II furcation defects in terms of decreased PPD, increased CAL gain, and radiographic bone fill in furcation defects.
To evaluate the efficacy of osteon II+PRF membrane+systemic dosage of CQ in Class II furcation defects in terms of decreased PPD, increased CAL gain, and radiographic bone fill in furcation defects.
To evaluate the efficacy of osteon II+PRF membrane+CQ used both locally and systemically in Class II furcation defects in terms of decreased PPD, increased CAL gain, and radiographic bone fill in furcation defects.
To compare the efficacy of CQ (Hadjod) as an adjunct to PRF and osteon II when used systemically, locally, and in combination for Class II furcation defects, in terms of decreased PPD, increased CAL gain, and radiographic bone fill in furcation defects.
Null hypothesis: The combination application of CQ adjunct to bone graft (Osteon II) in the Class II furcation management protocol compared to bone graft alone is having same regeneration qualities.
Alternative hypothesis: The combination application of CQ adjunct to bone graft (Osteon II) in the Class II furcation management protocol is efficacious and having superior regeneration qualities.
Materials and Methods
A randomised parallel-group trial will be conducted in the Department of Periodontics at Sharad Pawar Dental College, for two years, from April 2, 2024, to March 2026. The study will adhereto the principles outlined in the 1964 Helsinki Statement and its subsequent updates, as well as equivalent ethical standards and the policies of the Institutional and national research committees governing all processes involving human subjects. The Institutional Ethics Committee of the Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Wardha, has approved this research under reference number DMIMS(DU)/IEC/2022/1123, dated July 4, 2022. This trial is registered with the Clinical Trial Registry of India, Registration Number CTRI/2023/01/049282. The outpatient Department of Periodontics at Sharad Pawar Dental College, Sawangi (Meghe), Wardha, will select 60 systemically healthy patients aged 30 to 50 years with moderate to severe chronic periodontitis and Class II furcation deformities, either buccally or lingually, using the established guidelines. Written informed consent will be obtained after patient selection, following the norms outlined below.
Inclusion criteria:
Class II furcation defects in the mandibular molars with buccal or lingual involvement.
A horizontal furcation probing depth (HDD) of less than 3 mm.
The proximal bone height of the experimental tooth must be coronal to the inter-radicular bone level.
A sufficient amount of keratinised tissue must be present.
The experimental tooth must have undamaged surfaces near the furcation area and a typical response to the electric pulp test.
The gingival margin of the experimental tooth must be coronal to the furcation fornix.
Patients must not have any systemic illnesses.
Radiographic evidence of molar furcation involvement (buccal-lingual, mesiobuccal, or distobuccal) is required.
Exclusion criteria:
Patients who do not adhere to a periodontal maintenance program.
Subjects who smoke or use tobacco products in any form.
Subjects with mobility in a single tooth.
Allergies to the graft, local anesthetics, chlorhexidine, antibiotics, or analgesics.
Any previous periodontal regeneration procedures performed at the site in question.
Pregnant or lactating females.
Patients with infectious diseases such as hepatitis, Human Immunodeficiency Virus (HIV), or tuberculosis.
A carefully designed chart will record personal history, including nutritional status, oral hygiene practices, systemic background, and gingival and periodontal status. The subjects will be examined using a mouth mirror, William’s calibrated periodontal probe, and Naber’s probe for checking furcation involvement.
Sample size calculation: The sample size is determined using the following formula:
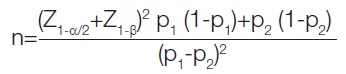
Proportion of outcome (p1)=0.83 [23]
Proportion of outcome (p2)=0.75
Level of significance (α)=0.05
Power (1-β)=0.80
Z alpha value=1.96
Z beta value=0.84

Sample size=36
Attrition=20%
Total sample size=60
Since, there are four groups, each group will consist of 15 samples.
Study Procedure
After a thorough examination and diagnosis, scaling and root planing will be performed under local anaesthesia. A review will be conducted six weeks after the scaling and root planing to assess the patient’s response and determine whether periodontal surgery is required. A specially designed occlusal acrylic stent will be fabricated to standardise angulations and probe position.
A total of 60 subjects with Class II furcation defects will be included in the study. After initial therapy and before surgery, the selected defects will be randomly assigned using a computer-generated randomisation technique, with a randomised table to create four groups, each containing 15 defects, as outlined below. A re-evaluation will be performed after nine months of therapy to estimate outcomes.
Group A: Osteon II+PRF membrane in Class II furcation defects+antibiotic coverage
Group B: Osteon II+PRF membrane+CQ graft (locally) in Class II furcation defects+antibiotic coverage
Group C: Osteon II+PRF membrane+systemic dosage of CQ in Class II furcation defects+antibiotic coverage
Group D: Osteon II+PRF membrane+CQ graft (locally)+systemic dosage of CQ in Class II furcation defects+antibiotic coverage
Method of preparation of CQ (Soxhlet method) [25]: The CQ stem will be collected from MGACH and the Research Centre Garden in Salod, Wardha’s local area and it will be authenticated at the Department of Rasashashtra and Bhaishajya Kalpana (BK). The stem portion of CQ will be shade-dried at room temperature. Subsequently, it will be subjected to size reduction to obtain a coarse powder of the desired particle size. The powdered material will be subjected to an aqueous extraction, which will then be dried to obtain a granular structure. The appearance of a colorless solvent in the siphon tube will be taken as the endpoint of the extraction. The extracts will be concentrated by distillation. The yield will be 10.8% and 14.8% w/w for the petroleum ether and methanol extracts, respectively.
Sterilisation of the CQ graft: A hot air oven will be used to sterilise the CQ graft material.
The Plaque Index (PI), Papillary Bleeding Index (PBI), Probing Pocket Depth (PPD), Relative Clinical Attachment Level (R-CAL), and comparative marginal gingival level (R-GML) will be documented as clinical measurements. Hard tissue parameters will include the distance from the roof-crest of the furcation to the crest of bone at the furcation entry, as well as the Cementoenamel Junction to the Mesial or Distal interdental bone crest (CEJ-D, CEJ-M). All clinical measurements will be taken on the day of surgery and at three and six months, postoperatively. The primary endpoint of the study will be radiographic bone fill, with secondary outcomes including gains in Clinical Attachment Level (CAL) and Probing Pocket Depth (PPD).
Before starting the treatment, participants will be advised to gargle for one minute with a 0.2% chlorhexidine gluconate solution. During the operation, asepsis will be maintained. The area will be anesthetised, and a flap incision will be made. Intra-crestal incisions will be placed on the lingual or buccal aspects of the affected tooth using surgical blades no. 12 or 15.
A full-thickness mucoperiosteal flap will be raised at the affected site using an osseous elevator to reveal the underlying defective margin (24 G Hu-Friedy, USA). During the removal of granulation tissue, considerable caution will be exercised to prevent damage to the flap or loss of the papilla.
Debridement and root surface management: To remove the pocket epithelium, the subsurface of the flap will be carefully curetted. Hand instruments, ultrasonic instruments, and furcation curettes will be used to debride the denuded root surface and the vault of the furcation. The root surfaces and furcation vaults will be planed until a smooth, firm consistency is achieved.
Intraoperative measurements of the horizontal and vertical defect depths at the furcation site will be taken following irrigation with physiologic saline solution and achieving haemostasis. The University of North Carolina (UNC)-15 probe will be inserted vertically into slots cut into the acrylic stent, and the reading taken from the stent’s inferior border to the gingival margin will be recorded as the Relative Gingival Marginal Level (R-GML). The distance between the base of the pocket and the stent’s lower border will be measured using the UNC-15 probe to determine the Relative Clinical Attachment Level (R-CAL). The Vertical Probing Depth (V-PPD) will be computed from the base of the pocket to the gingival margin (PPD). A curved, colour-coded Naber’s probe with 0-3, 3-6, and 6-9 mm markings will be used to measure the Horizontal Probing Depth (HPD) in the furcation area. Using the UNC-15 probe, the Width of the Keratinised Gingiva (WKG) will be estimated from the apical most point of the mucogingival interface to the crest of the gingival edge. If, the furcation defect depth is ≤3 mm both vertically and horizontally, the final eligibility of the patient for inclusion in the study will be confirmed after the intra-surgical measurements. The sites will then be randomly assigned to one of the four groups.
Postoperative care: In conjunction with the usual antibiotic treatment, patients in Group C and Group D will be given C. quadrangularis capsules (250 mg twice daily) for 50 days postoperatively. All participants will be instructed to gargle with 0.2% chlorhexidine gluconate twice daily for one minute each time for 4-6 weeks. After seven days, the periodontal dressing and stitches will be removed, and healing will be assessed. The individuals will be summoned back after one, three, and six months for follow-up.
Outcomes
I) Indices: The full-mouth Plaque Index (PI) will be measured before anaesthesia, at baseline, and after six months using the PI [26], while gingival inflammation will be assessed using the Papillary Bleeding Index (PBI) [27].
II) Probing measurements: Probing depth will be measured with a surgical stent and a UNC-15 calibrated periodontal probe (University of North Carolina, Hu-Friedy). At three locations on each furcation surface, the Vertical Probing Pocket Depth (V-PPD), Relative Clinical Attachment Level (R-CAL), and Relative Gingival Margin Level (R-GML) will be recorded: distal, mesial line angle, and mid-buccal or mid-lingual surface. The deepest measurement per defect will be included for result analysis. All probing measurements will be recorded at baseline, 30 days, and 60 days after surgery.
III) Radiographic measurements: Measurements for the vertical component will be carried out using a sagittal view with Cone Beam Computed Tomography (CBCT) as follows:
First, the Cementoenamel Junction (CEJ) will be identified, and a horizontal line will be drawn from the mesial to the distal aspect of the tooth.
A perpendicular line will be drawn from the middle of the tooth to the middle of the furcation, extending to the alveolar crest.
The distance between the alveolar crest and the point where this line meets the first line will be measured.
Horizontal component measurements will be performed in an axial view using Cone Beam Computed Tomography (CBCT) as follows:
A line will be drawn from the most buccal end of one root to the other.
A perpendicular line will then be drawn from the center of the first line until it meets the bone trabeculae at the crest [28].
1) Horizontal Defect Depth (HDD): The furcation defect will be measured horizontally at its deepest area with a UNC-15 probe. Another probe may be inserted at the prominence of the root surface as a reference to bridge the first probe [29].
2) Vertical Defect Depth (VDD): The furcation defect will be measured vertically at the deepest area of the furcation fornix, which will serve as a fixed reference point. If, the furcation defect depth is ≤3 mm both vertically and horizontally, the final eligibility of the patient for inclusion in the study will be confirmed [29].
Maintenance care: Subjects will be re-evaluated at baseline and nine months postsurgery. Oral hygiene instructions and full-mouth oral prophylaxis using ultrasonic instruments will be provided at each follow-up visit. Additionally, standardised radiographs and CBCT will be taken.
Statistical Analysis
The data will be analysed using the Statistical Package for Social Sciences (SPSS), version 27. Power calculations will be performed using the Student’s t-test. A p-value of less than 0.05 will be considered significant.
[1]. Chowdhary Z, Mohan R, Furcation involvement: Still a dilemmaIndian Journal of Multidisciplinary Dentistry 2017 7(1):34 [Google Scholar]
[2]. Bjorn Al, Hjort P, Bone loss of furcated mandibular molars. A longitudinal studyJ Clin Periodontol 1982 9:402-08. [Google Scholar]
[3]. Ghangurde AA, Ganji KK, Bhongade ML, Sehdev B, Role of chemically modified tetracyclines in the management of periodontal diseases: A reviewDrug Res (Stuttg) 2017 67(05):258-65. [Google Scholar]
[4]. Raja S, Nath G, Emmadi P, Ahathya R, Treatment of an isolated furcation involved endodontically treated tooth-A case reportJournal of Conservative Dentistry 2007 10(4):129-33. [Google Scholar]
[5]. Melcher AH, On the repair potential of periodontal tissuesJ Periodontol 1976 47:256-60. [Google Scholar]
[6]. Sanyal A, Ahmad A, Sastry M, Calcite growth in Cissus quadrangularis plant extract, a traditional Indian bone-healing aidCurr Sci 2005 89:1742-45. [Google Scholar]
[7]. Justin SR, Baby J, Pharmacognostic and traditional properties of Cissus quadrangularis Linn- An overviewInt J Pharm Bio Sci 2011 2:131-39. [Google Scholar]
[8]. Nayar M, Pharmalogical study of the stem of Cissus quadrangularis LinnJ Sci Ind Res 1959 18:253 [Google Scholar]
[9]. Jain M, Nivedhitha MS, Deepak S, Rajesh KS, A novel natural product for bone regeneration in dentistry--A reviewJ Evolution Med Dent Sci 2020 9(38):2833-39. [Google Scholar]
[10]. Zaffe D, Traversa G, Mozzati M, Morelli F, D’Angeli G, Behavior of aqueous nanocrystalline hydroxyapatite in oral bone regenerationJ Appl Biomater Biomech 2011 9(1):19-25. [Google Scholar]
[11]. Nery EB, Lee KK, Czajkowski S, Dooner JJ, Duqqan M, Ellinger RF, A veteran administration cooperative study of biphasic calcium phosphate ceramic in periodontal osseous defectsJ Periodontol 1990 61:737-44. [Google Scholar]
[12]. Nery EB, LeGeros RZ, Lynch KL, Lee K, Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta-TCP in periodontal osseous defectsJ Periodontol 1992 63:729-35. [Google Scholar]
[13]. Mangano C, Sinjari B, Shibli JA, Mangano F, Hamisch S, Piattelli A, A human clinical, histological, histomorphometrical, and radiographical study on biphasic ha-beta-tcp 30/70 in maxillary sinus augmentationClin Implant Dent Relat Res 2015 17(3):610-08. [Google Scholar]
[14]. Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D, Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigsClin Oral Implants Res 2006 17(3):237-43. [Google Scholar]
[15]. Dorozhkin SV, Calcium orthophosphates in dentistryJ Mater Sci Mater Med 2013 24(6):1335-63. [Google Scholar]
[16]. Dorozhkin SV, Self-setting calcium orthophosphate formulationsJ Funct Biomater 2013 4(4):209-311. [Google Scholar]
[17]. Guo D, Xu K, Han Y, The in situ synthesis of biphasic calcium phosphate scaffolds with controllable compositions, structures, and adjustable propertiesJ Biomed Mater Res A 2009 88(1):43-52.10.1002/jbm.a.31844 [Google Scholar] [CrossRef]
[18]. Lal N, Dixit J, Biomaterials in periodontal osseous defectsJ Oral Biol Craniofac Res 2012 2(1):36-40. [Google Scholar]
[19]. Choukroun J, Adda F, Schoeffer C, Vervelle A, PRF: An opportunity in perio-implantologyImplantodontie 2000 42:55-62. [Google Scholar]
[20]. Agrawal M, Agrawal V, Platelet rich fibrin and its applications in dentistry- A review articleNational Journal of Medical and Dental Research 2014 2:51-58. [Google Scholar]
[21]. Maiti SK, Saravanan B, Singh GR, Kumar N, Hoque M, Lal J, Evaluation of the Herb, Cissus quadrangularis in accelerating the healing process of femur osteotomies in dogsJ Appl Anim Res 2007 31(1):47-52. [Google Scholar]
[22]. Managutti A, Shah D, Patel J, Puttanikar N, Shah D, Managutti S, Evaluation of clinical efficacy of cissus quadrangularis in pain management and bone healing after implant placement - A pilot studyMedico Research Chronicles 2015 2(5):618-25. [Google Scholar]
[23]. Jain A, Dixit J, Prakash D, Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: Exploratory clinical trialJ Int Acad Periodontol 2008 10(2):59-65. [Google Scholar]
[24]. Shirmohammadi A, Roshangar L, Chitsazi MT, Pourabbas R, Faramarzie M, Rahmanpour N, Comparative study on the efficacy of Anorganic Bovine Bone (Bio-Oss) and Nanocrystalline Hydroxyapatite (Ostim) in maxillary sinus floor augmentationInt Sch Res Notices 2014 2014:967091Erratum in: Int Sch Res Notices. 2017 May 11;2017:7258513. PMCID: PMC489728110.1155/2014/96709127382621 [Google Scholar] [CrossRef] [PubMed]
[25]. Swamy AH, Kulkarni RV, Koti BC, Gadad PC, Thippeswamy AH, Gore A, Hepatoprotective effect of cissus quadrangularis stem extract against rifampicin-induced hepatotoxicity in ratsIndian J Pharm Sci 2012 74(2):183-87.PMCID: PMC354634010.4103/0250-474X.10385923326004 [Google Scholar] [CrossRef] [PubMed]
[26]. Löe H, The gingival index, the plaque index and the retention index systemsJ Periodontol 1967 38(6):610-16.10.1902/jop.1967.38.6.6105237684 [Google Scholar] [CrossRef] [PubMed]
[27]. Alfiandini RN, Prahasanti C, Wibisono PA, Papillary bleeding index in public health service on gingival inflammationInternational Journal for Pharmaceutical Research Scholars 2020 12(4):1575-78.10.31838/ijpr/2020.12.04.225 [Google Scholar] [CrossRef]
[28]. Srebrzyńska-Witek A, Koszowski R, Różyło-Kalinowska I, Piskórz M, CBCT for estimation of the cemento-enamel junction and crestal bone of anterior teethOpen Med (Wars) 2020 15(1):774-81.PMCID: PMC771209110.1515/med-2020-021133336035 [Google Scholar] [CrossRef] [PubMed]
[29]. Dambhare A, Bhongade ML, Dhadse PV, Sehdev B, Ganji KK, Thakare K, A randomized controlled clinical study of autologous Platelet Rich Fibrin (PRF) in combination with HA and Beta-TCP or HA and Beta-TCP alone for treatment of furcation defectsJ Hard Tissue Biology 2019 28(2):185-90.10.2485/jhtb.28.185 [Google Scholar] [CrossRef]