Introduction
Third molar extraction is a common surgical procedure that can have unintended consequences, such as periodontal disease that results in bone loss on the distal part of the second molar. By halting the atrophy of the alveolar ridge, socket and ridge preservation procedures enable restorations that are both functional and aesthetically acceptable. Maintaining the volume of the alveolar socket after tooth extraction is the primary objective of alveolar management, as it prevents substantial resorption and facilitates socket repair. Numerous methods have been reported on socket healing following tooth extractions. Grafting materials may promote healing of the post-extraction socket in three separate ways: osteogenesis, osteoinduction and osteoconduction.
Need of the study
This study is necessary because managing bony defects after the extraction of impacted mandibular third molars is critically dependent on successful interventions. Third molars that are impacted often need to be surgically removed, resulting in bony defects that require proper regeneration for the best possible outcome after surgery. An important consideration in this process is the selection of grafting materials. It is imperative to comprehend the relative effectiveness of dentine graft and Platelet-rich Fibrin (PRF) to assist clinicians in deciding the best treatment option. Both PRF and dentine graft have shown promise in fostering bone regeneration; however, to develop evidence-based guidelines for clinical practice, a direct comparison using a rigorous split-mouth study is essential.
Aim
To compare and evaluate outcomes of dentine graft against PRF in postoperative impacted mandibular third molar bony defects.
Materials and Methods
A prospective randomised split-mouth study will be conducted in the Department of Oral and Maxillofacial Surgery at Sharad Pawar Dental College, DMIHER, Sawangi, Wardha, Maharastra, India from April 2023 to January 2025. After the extraction of the 3rd molar, the patient will be instructed to bite on a sterile swab while waiting for the biomaterials to be ready. Following the examination of the extraction socket, the particulate dentine graft and liquid PRF preparation will be made and then placed in the extraction socket. The patient will then be instructed to use chlorhexidine mouth rinse (0.2%) twice a day in place of mechanical plaque management in the treated region for one week, as part of the postoperative regimen. Postoperative pain, wound healing, bone regeneration, and complications, if any will be assessed at a specified interval. Fisher’s-exact test will be used for statistical analysis, and the level of significance will be set at p<0.05.
Introduction
In the field of oral and maxillofacial surgery, impacted Lower Third Molar (LTM) extraction surgery is a relatively common procedure, whether it is performed for therapeutic or preventive purposes [1]. The incidence of impaction ranges from 66% to 77% [2]. A common surgical procedure that may have unintended repercussions is third molar extraction. One such result is periodontal disease, which is characterised by bone loss in the distal region of the second molar [3].
Many writers advocate for the use of regenerative procedures as adjunctive therapies, demonstrating that, in contrast to extraction without biomaterial grafting, the use of bone substitutes with resorbable or non resorbable membranes is a viable way to cure post-extraction deficiencies [4-6].
Various biomaterials have been administered to these post-extraction locations to enhance or promote bone growth. However, because autogenous bone is the sole material that satisfies the requirements of osteogenesis, osteoconduction, and osteoinduction, it is still regarded as the preferred material for bone regeneration [7,8]. However, because of its restricted availability and related morbidity at the donor site, it has several drawbacks [9].
Dentin has been studied as a potential biomaterial for bone repair in recent years. Dentin graft was first used in implant dentistry in 2010, where the dentin was retrieved from the patient’s extracted teeth [10]. Its 20% organic content is made up of a 90% type I collagen network, 10% non collagenous proteins (such as osteocalcin, osteonectin, sialoprotein, and phosphoprotein, which aid in bone calcification), and 10% growth factors (such as insulin-like growth factor and bone morphogenetic proteins, or Biomechanical Preparations (BMP), which give teeth their osteoinductive qualities) [11,12]. The remaining 10% is made up of water. However, it lacks the osteogenic potential of autogenous bone and its availability is restricted based on the state of extracted teeth [13].
In the context of sinus lifting and alveolar preservation, several studies have employed dentin for bone regeneration in implant dentistry [14-18]. A clinical case report from 2015 detailed the use of autogenous dentin for the alveolar preservation of an upper right third molar socket. During a one-year follow-up micro- Computed Tomography (CT) scan, bone growth was observed in the grafted area, as seen in periapical radiographs and microcomputerised tomography [19].
The initial three months of healing in an extraction socket involve the greatest amount of dimensional change in both bone and soft-tissue. Autologous PRF is one of the primary substances studied and stimulates new bone formation and wound healing. Its therapeutic approach is founded on the idea that growth factors, which are universal initiators of healing events, might accelerate healing. The growth factors included in PRF are well known. When they are administered to bony defects, these factors are thought to have the ability to stimulate tissue repair by accelerating chemotaxis, mitogenesis, angiogenesis, and collagen matrix formation [20]. Despite its benefits, the process of making PRF requires an expensive centrifuge machine and is tedious.
Nowadays, a variety of biomaterials are utilised to regenerate bone; however, autogenous bone is regarded as the ideal material due to its osteogenic, osteoconductive, and osteoinductive qualities. Autogenous dentin has been reported to be used as a graft material in periodontal pockets in recent years. This grafting technique is commonly employed in procedures like socket preservation, ridge augmentation, and sinus floor augmentation to enhance bone volume and support dental implant placement. The use of autogenous dentin offers advantages such as biocompatibility and a low-risk of rejection, making it a promising option in the field of regenerative dentistry [21]. The present study aims to evaluate and compare the outcomes of dentine graft over PRF in post-third molar extraction bony defects.
Primary objectives:
To evaluate the outcomes of dentine graft in postoperative impacted mandibular third molar bony defects.
To evaluate the outcomes of PRF in postoperative impacted mandibular third molar bony defects.
To compare the outcomes of dentine graft against PRF in postoperative impacted mandibular third molar bony defects.
Secondary objectives:
Evaluate the long-term stability and success rates of dental implants placed in sites augmented with autogenous dentin grafts.
Compare the outcomes of autogenous dentin grafts with PRF in terms of bone volume preservation.
Analyse the impact of autogenous dentin grafts on the surrounding soft-tissues, including gingival health and aesthetics.
Review of Literature
Long-term clinical studies have demonstrated stable outcomes and high patient satisfaction rates, affirming the safety of dentin grafts in supporting and regenerating natural bone structures [14,21]. The study conducted at the Complutense University of Madrid aimed to evaluate the effectiveness of autogenous dentin grafts in promoting periodontal regeneration and healing after impacted LTM extraction. A total of 15 patients underwent bilateral LTM extraction, with autogenous dentin grafts used on the test side and no graft on the control side. The autogenous dentin graft group showed superior clinical insertion levels and reduced Probing Depth (PD) compared to the control group. These results suggest that autogenous dentin grafts can be a useful graft material for regenerating periodontal defects after impacted LTM extraction [22].
A 2021 review done by Um IW et al., reports that dentin grafts are generally well-tolerated by the host, exhibiting minimal immunogenic responses and inflammatory reactions [23]. Furthermore, Zhang S et al., have conducted comparative analysis against traditional bone graft materials, revealing dentin’s comparable or even superior biocompatibility [24].
A review conducted in Embase, Medical Literature Analysis and Retrieval System Online (MEDLINE), and the Cochrane Central Register of Controlled Trials included all randomised controlled trials. Postoperative discomfort, soft-tissue healing, bone density, changes in the horizontal and vertical ridge dimensions, and histologic examination were all systematically reviewed. Changes in mesial and distal bone height, as well as alveolar osteitis, were the subjects of the meta-analysis. After tooth extraction, PRF should be considered due to its potential utility. Further high-quality experiments are needed to precisely assess PRF’s involvement [25].
Materials and Methods
A prospective randomised split-mouth study will be conducted in the Department of Oral and Maxillofacial Surgery, Sharad Pawar Dental College, DMIHER, Sawangi, Wardha, Maharastra, India for a period of two years, from April 2023 to January 2025. A written Informed consent will be taken from the subjects. Ethical approval has been received from Datta Meghe Institute of Higher Education and Research, Sawangi, Wardha with IEC reference number- DMIHER(DU)/IEC/2023/.
Inclusion criteria:
All patients aged 18 years and above with impacted bilateral mandibular third molars are indicated for extractions (which are not required to be extracted simultaneously).
A medical history devoid of any pharmacological therapy that could introduce variables into the experiments.
Exclusion criteria:
All patients who are not willing to be a part of the study.
Patients with diabetes mellitus, coronary artery disease, peripheral vascular diseases, and cancer.
Patients on long-term corticosteroid therapy.
Patients with immunocompromised status.
Pregnant and lactating females.
Patients with periapical infections and cysts.
Sample size calculation: Sample size formula using mean difference:
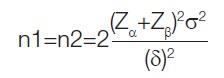
Where, Zα=1.96, α=Type I error at 5%, Zβ =0.84 at (1-β)=Power at 80%, σ=standard deviation, γ=True Difference in means
Mean soft-tissue index (Before) Treatment group=4.8 at 3rd day and
Mean soft-tissue index (After) Treatment group=4.1 at 21 day [26]
Difference in mean=0.7.
Standard Deviation=0.6
Minimum sample size N=2*(1.64+0.84)2(0.6)2/(0.7)2=9.
Null and Alternate Hypotheis:
Null hypothesis (H0): “There is no significant difference in bone regeneration or healing outcomes between the use of dentine graft and Platelet-rich Fibrin (PRF) in postoperative impacted mandibular third molar bony defects.” This means that the study assumes both dentine graft and PRF will lead to similar outcomes in terms of promoting healing, bone regeneration, and postoperative recovery.
Alternative hypothesis (H1): “There is a significant difference in bone regeneration or healing outcomes between the use of dentine graft and Platelet-rich Fibrin (PRF) in postoperative impacted mandibular third molar bony defects.” This implies that one treatment method either dentine graft or PRF results in superior outcomes in terms of promoting healing, bone regeneration, or reducing complications post-extraction.
Study Procedure
Group IA- Dentin graft: Sterile gauze will be used to remove any remaining soft-tissue after the tooth is removed whole or divided using an odonto-section, and compressed air will be used to dry it. The tooth will next be placed in a dentin grinding machine (Smart Dentin Grinder®, KometaBio, Bioner, Barcelona, Spain) and ground for three seconds, following the manufacturer’s recommendations. A particle size of 300-1200 μm will be achieved by sieving it for 20 seconds after it has been grounded. The acquired material will be combined for 12 minutes in a sterile flask with 0.5% sodium hydroxide and 20% ethanol to remove any remaining organic matter, germs, or toxins from the dentin and then, will be immersed in saline for three minutes; the dentin graft will be taken out and positioned [27].
Group II PRF: To obtain the PRF, the subject’s blood will be drawn into 9 mL Dry Vacutube tubes without the addition of any additives. Three tubes will be gathered and then put in the horizontal centrifuge (a tube full of water will be added to keep the balance throughout the two minutes of centrifuging at 3300 rpm). When this procedure is finished, the leftover blood components below and an orange-coloured region in the tube (PRF) will be seen. After that, the tubes will be cautiously opened to prevent the material from becoming homogenised. A 20 mL syringe and an 18 G hypodermic needle will be used to draw 5 mL of PRF from the tubes (Injex® Brazil).
Primary outcomes:
Soft-tissue healing: This will be measured by clinical examination of the socket closure and mucosal healing at specified intervals. (Post-op day 2nd, 1 week, 3 months).
Bone regeneration: This will be assessed via radiographic imaging {e.g., Cone Beam Computed Tomography (CBCT)} to evaluate the amount of new bone formation and density within the extraction socket at specified intervals (e.g., 3 months, 6 months).
Postoperative pain and discomfort: This will be evaluated using a standardised pain scale {e.g., Visual Analogue Scale (VAS)} to measure patient-reported pain levels at different time points post-extraction (e.g., 24 hours, 3 days, 7 days) [28].
Incidence of complications: Including infection, dry socket (alveolar osteitis) will be evaluated.
Secondary outcomes:
Implant stability: This will be assessed through CBCT and peri-implant bone levels immediately post-surgery, 6 months, 12 months, and annually thereafter.
Implant success rate: This will be measured by criteria such as lack of implant mobility, absence of peri-implant radiolucency, and lack of persistent pain or infection at an Interval of 6 months and 12 months.
Bone volume preservation: This will be measured by radiographic analysis (CBCT or panoramic X-rays) to assess bone height and width immediately post-surgery, 3 months, 6 months, and 12 months.
Gingival health: This will be Assessed using indices such as the Gingival Index (GI) and Plaque Index (PI). Intervals of: one month, three months, six months, and 12 months.
Gingival aesthetics: This will be assessed clinically at intervals of three months, six months, and 12 months.
Statistical Analysis
For statistical evaluation the R Studio Version 4.3.1 will be used. TheFisher-exact test will be used for statistical analysis, and the level of significance will be set at p<0.05.
[1]. Coleman M, McCormick A, Laskin DM, The incidence of periodontal defects distal to the maxillary second molar after impacted third molar extractionJ Oral Maxillofac Surg 2011 69(2):319-21. [Google Scholar]
[2]. Ge J, Yang C, Zheng J, Hu Y, Autogenous bone grafting for treatment of osseous defect after impacted mandibular third molar extraction: A randomized controlled trialClin Implant Dent Relat Res 2017 19(3):572-80. [Google Scholar]
[3]. Kan KW, Liu JK, Lo EC, Corbet EF, Leung WK, Residual periodontal defects distal to the mandibular second molar 6-36 months after impacted third molar extractionJ Clin Periodontol 2002 29(11):1004-11. [Google Scholar]
[4]. Richardson DT, Dodson TB, Risk of periodontal defects after third molar surgery: An exercise in evidence-based clinical decision-makingOral Surg Oral Med Oral Pathol Oral Radiol Endod 2005 100(2):133-37. [Google Scholar]
[5]. Sammartino G, Tia M, Bucci T, Wang HL, Prevention of mandibular third molar extraction-associated periodontal defects: A comparative studyJ Periodontol 2009 80(3):389-96. [Google Scholar]
[6]. Barbato L, Kalemaj Z, Buti J, Baccini M, La Marca M, Duvina M, Effect of surgical intervention for removal of mandibular third molar on periodontal healing of adjacent mandibular second molar: A systematic review and Bayesian network meta-analysisJ Periodontol 2016 87(3):291-302. [Google Scholar]
[7]. Haugen HJ, Lyngstadaas SP, Rossi F, Perale G, Bone grafts: Which is the ideal biomaterial?J Clin Periodontol 2019 46(Suppl 21):92-102. [Google Scholar]
[8]. Moussa NT, Dym H, Maxillofacial bone grafting materialsDent Clin N Am 2020 64(2):473-90. [Google Scholar]
[9]. Hassan KS, Marei HF, Alagl AS, Does grafting of third molar extraction sockets enhance periodontal measures in 30- to 35-year-old patients?J Oral Maxillofac Surg 2012 70(4):757-64. [Google Scholar]
[10]. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, Development of a novel bone grafting material using autogenous teethOral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2010 109(4):496-503. [Google Scholar]
[11]. de Oliveira GS, Miziara MN, Silva ER, Ferreira EL, Biulchi AP, Alves JB, Enhanced bone formation during healing process of tooth sockets filled with demineralized human dentine matrixAust Dent J 2013 58(3):326-32. [Google Scholar]
[12]. Kabir MA, Murata M, Akazawa T, Kusano K, Yamada K, Ito M, Evaluation of perforated demineralized dentin scaold on bone regeneration in critical-size sheep iliac defectsClin Oral Implant Res 2017 28(11):e227-e35. [Google Scholar]
[13]. Gual-Vaqués P, Polis-Yanes C, Estrugo-Devesa A, Ayuso-Montero R, Marí-Roig A, López-López J, Autogenous teeth used for bone grafting: A systematic reviewMed Oral Patol Oral Cir Bucal 2018 23(1):e112-e19. [Google Scholar]
[14]. Lee JY, Kim YK, Yi YJ, Choi JH, Clinical evaluation of ridge augmentation using autogenous tooth bone graft material: Case series studyJ Korean Assoc Oral Maxillofac Surg 2013 39(4):156-60. [Google Scholar]
[15]. Pang KM, Um IW, Kim YK, Woo JM, Lee JH, Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine boneClin Oral Implant Res 2017 28(7):809-15. [Google Scholar]
[16]. Valdec S, Pasic P, Soltermann A, Thoma D, Stadlinger B, Rücker M, Alveolar ridge preservation with autologous particulated dentin-A case seriesInt J Implant Dent 2017 3(1):12 [Google Scholar]
[17]. Del Canto-Díaz A, De Elío-Oliveros J, Del Canto-Díaz M, Alobera-Gracia MA, Del Canto-Pingarrón M, Martínez-González JM, Use of autologous tooth-derived graft material in the post-extraction dental socket: Pilot studyMed Oral Patol Oral Cir Bucal 2019 24(1):e53-e60. [Google Scholar]
[18]. Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ, Clinical study of graft materials using autogenous teeth in maxillary sinus augmentationImplant Dent 2011 20(6):471-75. [Google Scholar]
[19]. Kabir A, Murata M, Kusano K, Akazawa T, Shibata T, Autogenous demineralized dentin graft for third molar socket regeneration-A case reportDentistey 2015 5(11):01-04. [Google Scholar]
[20]. Alzahrani AA, Murriky A, Shafik S, Influence of platelet rich fibrin on post-extraction socket healing: A clinical and radiographic studySaudi Dent J 2017 29(4):149-55.Epub 2017 Aug 2. PMCID: PMC563479510.1016/j.sdentj.2017.07.00329033524 [Google Scholar] [CrossRef] [PubMed]
[21]. Janjua OS, Qureshi SM, Shaikh MS, Alnazzawi A, Rodriguez-Lozano FJ, Pecci-Lloret MP, Autogenous tooth bone grafts for repair and regeneration of maxillofacial defects: A narrative reviewInt J Environ Res Public Health 2022 19(6):3690PMCID: PMC895550010.3390/ijerph1906369035329377 [Google Scholar] [CrossRef] [PubMed]
[22]. Sánchez-Labrador L, Martín-Ares M, Cortés-Bretón Brinkmann J, López-Quiles J, Martínez-González JM, Assessment of changes in the outcome of autogenous tooth grafts over time: A clinical study evaluating periodontal healing in bone defects after lower third molar removalJ Oral Maxillofac Surg 2024 :S0278-2391(24)00331-8Available from: https://doi.org/10.1016/j.joms.2024.05.006 [Google Scholar]
[23]. Um IW, Lee JK, Kim JY, Kim YM, Bakhshalian N, Jeong YK, Allogeneic dentin graft: A review on its osteoinductivity and antigenicityMaterials (Basel) 2021 14(7):1713PMCID: PMC803661110.3390/ma1407171333807291 [Google Scholar] [CrossRef] [PubMed]
[24]. Zhang S, Li X, Qi Y, Ma X, Qiao S, Cai H, Comparison of autogenous tooth materials and other bone graftsTissue Eng Regen Med 2021 18(3):327-41.Epub 2021 Apr 30. PMCID: PMC816972210.1007/s13770-021-00333-433929713 [Google Scholar] [CrossRef] [PubMed]
[25]. Pan J, Xu Q, Hou J, Wu Y, Liu Y, Li R, Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic reviewJ Am Dent Assoc 2019 150(9):766-78.ISSN 0002-8177, Available from: https://doi.org/10.1016/j.adaj.2019.04.025 [Google Scholar]
[26]. Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G, Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the healing of simple postextraction sockets: A split-mouth studyBiomed Res Int 2015 2015:369273Epub 2015 Jul 26. PMCID: PMC452991110.1155/2015/36927326273612 [Google Scholar] [CrossRef] [PubMed]
[27]. Binderman I, Hallel G, Nardy C, Yaffe A, Sapoznikov L, A novel procedure to process extracted teeth for immediate grafting of autogenous dentinJ Interdiscipl Med Dent Sci 2014 2(6):154 [Google Scholar]
[28]. McCormack HM, Horne DJ, Sheather S, Clinical applications of visual analogue scales: A critical reviewPsychol Med 1988 18(4):1007-19. [Google Scholar]