Introduction
Drug repurposing, also known as repositioning or drug reprofiling, involves identifying new applications for existing drugs, including those in early developmental stages or those that have been discontinued [1]. It is based on two fundamental insights. Firstly, many drugs have the ability to interact with multiple protein targets. Secondly, various diseases may share common genetic factors, molecular pathways, or clinical features [2].
Drug repurposing utilises two key methods: experimental approaches, which directly test existing drugs, and computational methods, which employ virtual tools like bioinformatics and network analysis to predict drug-target interactions based on public databases and molecular data [1].
Repurposing a drug has several key advantages. It is safer because these drugs already have known safety profiles. It is cost-effective, as it reduces the time and money required for development. It also taps into market potential more quickly, providing a competitive edge. Additionally, it offers a faster return on investment, which is appealing to both pharmaceutical companies and investors [3].
Periodontitis represents a progressive inflammatory disease initiated by specific bacterial pathogens, modulated by the host’s immune response, and influenced by genetic and environmental factors, ultimately leading to the destruction of the supporting structures of the teeth [4]. Lipopolysaccharides (LPS), along with other virulence factors produced by periodontal pathogens, activate host macrophages and other cells, such as fibroblasts, to release proinflammatory cytokines, including Tumour Necrosis Factor (TNF)- α, Interleukin (IL)-1β, and Prostaglandin E2 (PGE2) [5]. These cytokines trigger the production of Matrix Metalloproteinases (MMPs) by these cells, leading to the breakdown of collagen fibres in periodontal tissues [5,6]. Moreover, the proinflammatory cytokines stimulate the production of Receptor Activators of Nuclear Factor κB Ligand (RANK-L) on osteoblasts and T helper cells. RANK-L then binds to the RANK on osteoclast precursors, leading to the formation and maturation of osteoclasts. Fully developed osteoclasts are crucial for the degradation of alveolar bone in the context of periodontal disease [7].
Contemporary periodontal therapy adopts a multimodal strategy to manage periodontal disease. This approach integrates mechanical debridement for plaque and calculus removal, with the adjunctive use of antiseptics for bacterial control. In severe cases, surgical intervention may be necessary to enhance periodontal health [8].
In the field of periodontology, the scope of drug repurposing can be quite promising. The potential benefits in terms of cost, safety, and efficacy make this approach an exciting avenue for developing novel treatments for periodontal diseases and conditions. Investigating the repurposing of drugs for periodontal application adds an intriguing dimension to this field of research. Therefore, the present review aimed to explore the advantages of various drugs that have been repurposed for the treatment of periodontal conditions.
Metformin
Metformin is a second-generation biguanide with insulin-sensitising properties that is derived from the plant Galega officinalis. It has been used for the treatment of type 2 diabetes and as an adjunct to insulin therapy in type 1 diabetes since 1957 [9]. Metformin works by lowering blood glucose concentration through several mechanisms: increasing glucose uptake by the muscles, decreasing hepatic gluconeogenesis, and reducing glucose absorption in the intestine [10].
In recent years, it has been repurposed to benefit the treatment of various diseases, including neurorestorative conditions such as Alzheimer’s and Parkinson’s disease [11], and it acts as an antitumour agent for the treatment of hepatocellular carcinoma [12].
Currently, metformin is being explored as an adjunct to periodontal therapy [Table/Fig-1]. The main advantage of using metformin in this context is its ability to stimulate alveolar bone formation. Metformin can directly stimulate osteoblasts, partly by enhancing the expression of Runx2 and insulin-like growth factor 1. Additionally, it can reduce intracellular Reactive Oxygen Species (ROS) and apoptosis [13].
Summary of mechanism of action of metformin for periodontal application.
TNF-α: Tumour necrosis factor-α; IL: Interleukin
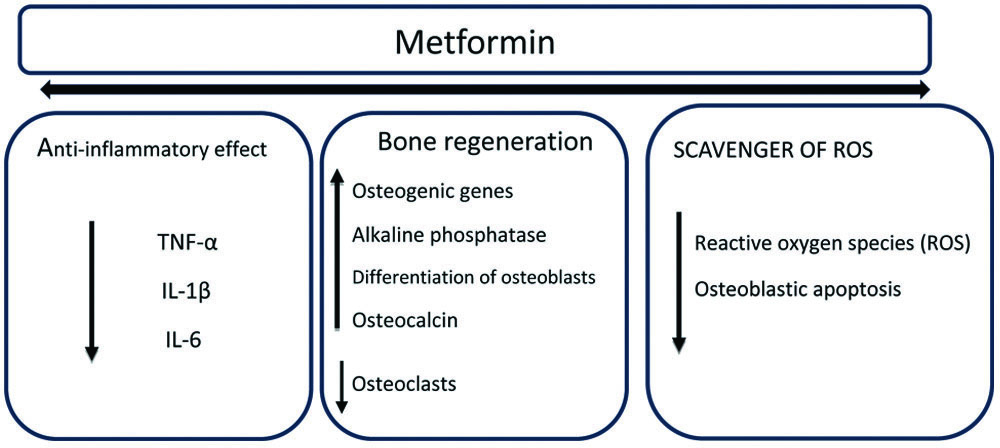
An in-vitro study in nondiabetic rats showed that the addition of metformin to a culture of rat adipose-derived multipotent mesenchymal stromal cells resulted in the differentiation of rat adipose tissue into bone-forming cells. Additionally, it was noted that metformin activates AMP-Activated Protein Kinase (AMPK), which is a crucial regulator of osteogenic differentiation [14]. Metformin also has an anti-inflammatory effect on various LPS-induced periodontal cells by suppressing the production of IL-6, IL-1β, and TNF-α through the activation of transcription factor-3 [15].
Several in-vivo studies on animal models with experimentally induced periodontitis have demonstrated positive effects of metformin on alveolar bone regeneration and wound healing [16,17]. A recent study that utilised a composite scaffold loaded with metformin in ligature-induced periodontitis in Sprague-Dawley male rats was analysed using Micro-Computed Tomography (CT) and histological methods, and it was shown to promote alveolar bone regeneration [16].
In humans, 1% Metformin has been successfully used as a local delivery agent as an adjunct in the treatment of chronic periodontitis [18]. It has been shown to improve clinical parameters such as Probing Depth (PD), Clinical Attachment Level (CAL), and reduction of Intrabony Defects (IBD) [19]. In the latest study, Metformin was used in combination with platelet-rich fibrin for the treatment of furcation defects. This combination therapy resulted in better clinical and radiographic outcomes, including a significant reduction in defect volume [20]. The osteogenic properties of Metformin are now being utilised due to its positive biostimulatory effect to improve osseointegration when coated on implants [21]. A systematic review on the use of Metformin as an adjunct to periodontal therapy has shown that it can improve IBD, reduce PD, and help improve CAL and peri-implant health [22].
Statins
Statins are a group of drugs commonly used in the treatment of hyperlipidemia. They are the most prescribed medications for reducing plasma cholesterol levels. These drugs work by inhibiting the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase [23]. They act by downregulating the production of mevalonate and its derivatives. In contrast to their hypolipidemic action, they stimulate nitric oxide production in endothelial cells and exhibit additional anti-inflammatory, anticoagulant, antioxidant, and antiarrhythmic activities [24]. Statins are currently being explored for repurposing in various medical contexts, including their potential use as anti-cancer agents [25], in the management of stroke [26], and even in the context of depression [27].
Lipophilic statins impact a regulatory pathway in monocytes that governs cytokine production. These statins are known to trigger diverse proinflammatory responses, both invitro and in-vivo [28]. Statins also activate several downstream signalling pathways, including Mitogen-Activated Protein Kinase (MAPK), p38, ERK1/2, and Nuclear Factor (NF) κB. This activation leads to increased production and release of both proinflammatory and anti-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-10, and IL-12p20 [29].
The use of statins in periodontal therapy is supported by their versatile properties, which encompass antimicrobial [30], anti-inflammatory [31], and bone-enhancing effects by enhancing the expression of three osteoinductive growth factors, namely Transforming Growth Factor beta 1 (TGF-beta 1), Bone Morphogenetic Protein-2 (BMP-2), and Vascular Endothelial Growth Factor (VEGF) [32] [Table/Fig-2].
Summary of mechanism of action of statins for periodontal application.
TNF-α: Tumour necrosis factor-α; IL: Interleukin; TGF-β1: Transforming growth factor beta 1; BMP-2: Bone morphogenetic protein-2; VEGF: Vascular endothelial growth factor; MMP: Matrix metalloproteinases
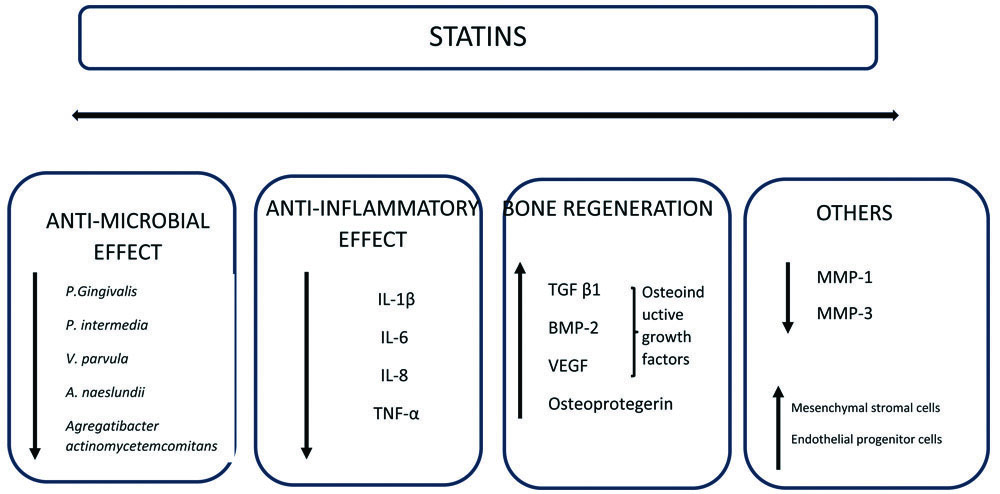
Additionally, statins are known to inhibit enzymes responsible for tissue breakdown, such as MMP [33]. They also promote the proliferation of mesenchymal stromal cells and endothelial progenitor cells [34]. Given these diverse therapeutic effects that go beyond merely controlling lipid levels, simvastatin, atorvastatin, and rosuvastatin are currently being repurposed for use in periodontal treatment [35-37].
Invitro experiments have shown that simvastatin and atorvastatin possess antimicrobial properties, effectively reducing Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans [38,39]. A study by Liu S et al., has shown that simvastatin can have a mild impact on cell metabolism and can enhance the expression of genes related to differentiation and osteogenesis in alveolar osteoblasts and periodontal ligament cells. These findings suggest that simvastatin may play a role in promoting the formation of alveolar bone and periodontal regeneration [40].
An in-vivo study involving experimentally induced periodontitis in rats demonstrated that the local administration of simvastatin could uphold heightened alkaline phosphatase activity and maintain a high level of osteoblastic function [41]. A systematic review of the effect of statins in experimentally induced periodontitis in animal models has shown that the utilisation of statins for periodontal purposes has a beneficial impact on various periodontal tissue parameters. These results substantiate the favourable clinical outcomes observed when statins are applied locally as an adjunct to both non surgical and surgical periodontal interventions [42].
In patients with chronic periodontitis, the combination of scaling and root planing along with the local application of simvastatin resulted in a more substantial reduction in PD, gain in CAL, and improved IBD fill [35]. In a comparative study, atorvastatin demonstrated a more significant enhancement in clinical parameters, including a higher percentage reduction in radiographic defect depth compared to simvastatin [36]. Rosuvastatin has also been shown to enhance bone formation at IBD, decreasing PD and increasing CAL [37]. Statins are becoming increasingly popular in the field of implant treatment. A systematic review has indicated that statins have a positive impact on enhancing new bone formation around implants and/or increasing bone-to-implant contact. Both local and systemic administration of statins appears to bolster osseointegration [43].
Bisphosphonates
Bisphosphonates, which are analogues of pyrophosphate, were initially synthesised in the 1800s. Their original use was primarily as corrosion inhibitors and complexing agents in various industries, including textiles, fertilisers, and oil [44]. Pyrophosphate has a strong binding affinity for bone minerals, particularly hydroxyapatite. This binding accounts for the specific pharmacological action of bisphosphonates on mineralised tissue, especially bone [45]. Following the discovery that bisphosphonates could effectively regulate the formation and dissolution of calcium phosphate, as well as influence mineralisation and bone resorption in animal models [46], they were further developed and employed for the treatment of bone-related disorders such as osteoporosis [47], Paget’s disease [48], and hypercalcaemia [49].
The primary types of adverse effects associated with some or all bisphosphonates include acute reactions, gastrointestinal disorders [50], and renal toxicity [51]. Additionally, osteonecrosis of the jaw is the most significant concern in clinical practice [52], with the mandible being the bone most affected. Patients frequently experience symptoms such as pain, halitosis, and difficulties in eating and speaking. Subtle changes in the health of periodontal tissue and unexplained infections may occur before the onset of this condition [53]. Practitioners should be mindful of this issue to avoid serious complications in patients undergoing long-term treatment with bisphosphonates.
Bisphosphonates exhibit a strong affinity for calcium ions, which results in their strong attraction to bone tissue. They employ a distinct mechanism to inhibit osteoclastic bone resorption; these compounds attach to hydroxyapatite binding sites on the bone’s surface, particularly in areas undergoing active resorption. When osteoclasts initiate the resorption of bone impregnated with bisphosphonates, the released bisphosphonate interferes with the osteoclast’s ability to form a ruffled border, thereby impairing bone resorption [54]. Additionally, bisphosphonates have the potential to counteract the activity of several MMP that participate in the degradation of structural elements within connective tissue [55,56]. As a result of their capacity to inhibit bone resorption and downregulate matrix metalloproteins, bisphosphonates are currently utilised as an adjunct in periodontal therapy [Table/Fig-3] [54,56].
Summary of mechanism of action of bisphosphonate for periodontal application [54,56].
MMP: Matrix metalloproteinases
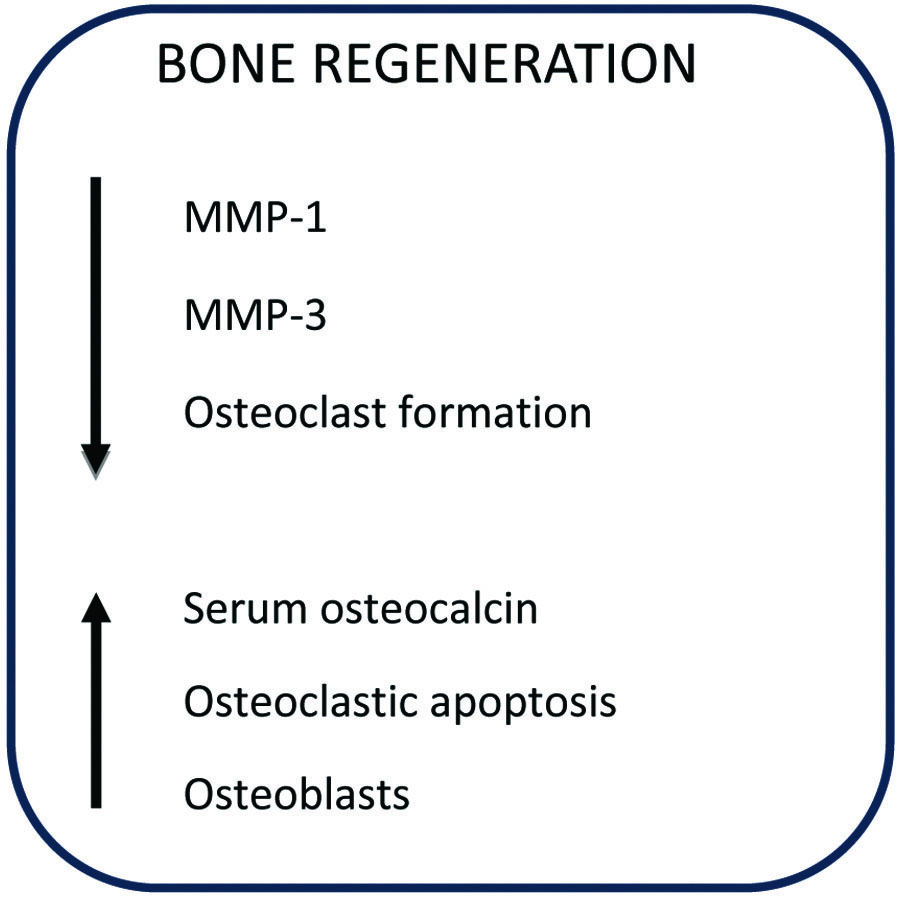
While bisphosphonates offer advantages when used as an adjunct to therapy, caution must be exercised regarding the dosage of the drug, as it can lead to adverse effects such as osteonecrosis [53]. Invitro investigations suggest a potential inhibitory effect of bisphosphonates on cells critical for bone homeostasis. These studies have shown a reduction in cell proliferation and collagen production among human gingival fibroblasts and osteoblasts [57,58]. However, another study suggests that as the drug concentration increases, there is a significant decrease in cell viability, rendering it cytotoxic; hence, lower concentrations of this drug should be considered to achieve therapeutic benefits [59].
Studies involving rats with experimentally induced periodontitis revealed that treatment with systemic bisphosphonates effectively prevented bone resorption, loss of bone mineral content, and morphologic changes of the osteoclasts [60,61]. Similarly, topically applied bisphosphonates have also demonstrated the ability to inhibit intraarticular alveolar bone loss in rats [62].
Clinical trials have investigated the effects of bisphosphonates on periodontal treatment. These studies involved either systemic administration of the drug or local delivery. The results suggested that systemic bisphosphonates, when used alongside non surgical periodontal therapy, improve clinical outcomes and bone density [63,64]. Due to concerns about potential side effects of systemically administered bisphosphonates, locally delivered bisphosphonates have been used to treat periodontitis. Local delivery of alendronate gel in patients with periodontitis has shown improvements in periodontal parameters, including bone fill and enhancements in furcation defects [65,66].
Systematic reviews have been conducted to evaluate the effectiveness of bisphosphonate therapy as an adjunct to scaling and root planing in the management of periodontitis. The results indicated that bisphosphonates are effective in managing periodontitis; however, the potential risk of osteonecrosis of the jaws and their clinical application remains a subject of debate [67,68]. Emerging research explores the application of alendronate-coated dental implants to enhance osseointegration. Preliminary findings indicate a potential increase in the rate of early bone formation around the implants [69].
Melatonin
Melatonin, initially recognised as a hormone that contracts melanophores, was first identified as a substance that lightens skin in frogs and fish. In 1917, Mccord CP and Allen FP noted that extracts from the pineal glands of cattle had a strong skin-lightening effect on frog skin [70]. This compound is synthesised by the pineal gland in the human brain and plays a role in modulating immune defense responses, regulating body weight, influencing reproductive processes, inhibiting tumour growth, and alleviating the effects of jet lag [71]. Beyond its established role in circadian rhythms, melatonin’s potential as an antioxidant and its therapeutic applications in diverse conditions, such as diabetes, cardiovascular diseases, and neurodegenerative disorders, warrant further exploration, including its use in periodontal therapy [Table/Fig-4] [72,73].
Summary of mechanism of action of melatonin for periodontal application [72,73].
TNF-α: Tumour necrosis factor-α; IL: Interleukin; CRP: C-reactive protein
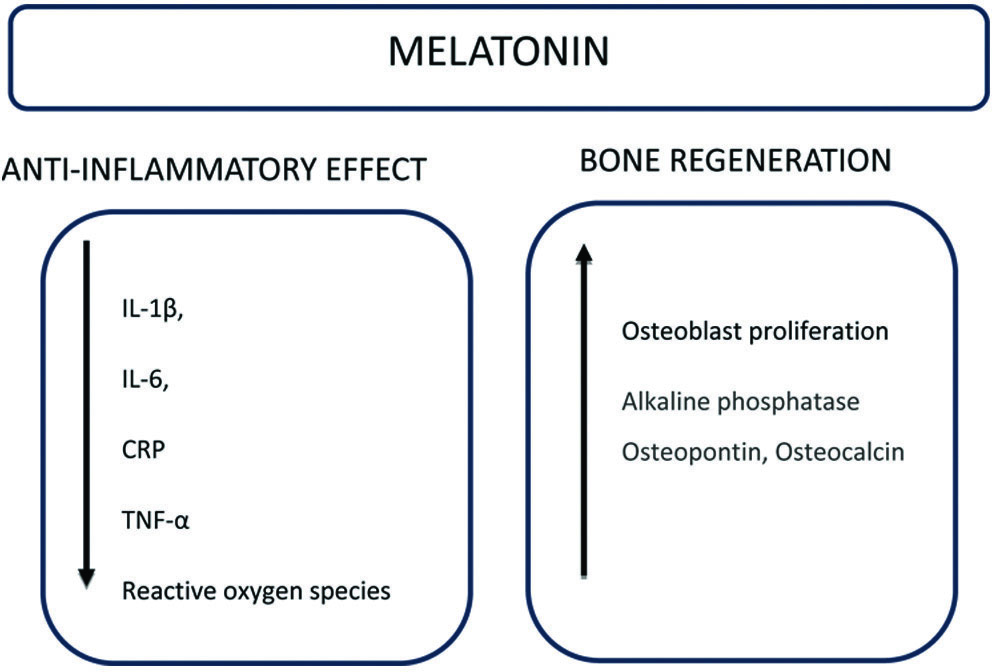
A recent systematic review has stated that melatonin levels in Gingival Crevicular Fluid (GCF), saliva, and serum of patients suffering from chronic periodontitis are lower, suggesting that melatonin may play a role in protecting tissues from damage caused by oxidative stress [74]. Treatment with intraperitoneally administered melatonin has been shown to significantly reduce alveolar bone resorption in experimentally induced periodontitis [75]. Additionally, a reduction in proinflammatory cytokines such as IL-1β, IL-6, C-reactive proteins, and TNF-α was observed in periodontitis patients with diabetes [76]. Melatonin also downregulates the receptor activator of NF kappa-B ligand/osteoprotegerin ratios and increases the levels of salivary acid phosphatase, alkaline phosphatase, osteopontin, and osteocalcin, which are indicative of bone formation [77,78].
Clinical trials suggest that dietary melatonin supplementation can enhance periodontal health by improving clinical parameters, including CAL and PD, with no reported adverse effects [79,80]. A recent study has shown that the application of 1% melatonin gel in IBDs results in significantly greater defect fill when used as an adjunct to non surgical periodontal therapy [73]. Additionally, a systematic review of the effect of melatonin on non surgical periodontal therapy has demonstrated that it enhances bone-to-implant contact and promotes new bone formation, potentially leading to improved success and long-term survival of implant treatments [81].
Studies evaluating the repurposed drug in periodontal therapy have been described in [Table/Fig-5] [18-21,35-37,41,60-62,64,65,75,79,80].
| Study | Study design | Materials and methods | Conclusion |
|---|
| Pradeep AR et al., (2013) [18] | Human clinical trial | 118 Intrabony Defects (IBD) treated with 0.5%, 1%, or 1.5% MF gel or placebo gel | MF improved IBD reduction. Maximum significance was achieved while using 1% MF |
| Pradeep AR et al., (2016) [19] | Human clinical trial | 65 patients with IBDs; 1% MF+SRP versus SRP and placebo gel | 1% MF showed significant improvement in the IBD |
| Swami RK et al., (2021) [20] | Split-mouth human clinical trial | 21 patients with Grade II furcation defects; PRF+1% MF versus PRF alone | 1% MF+PRF improved regenerative ability in Grade II furcation defects |
| Sharma H et al., (2021) [21] | Human clinical trial | 22 patients with missing mandibular posteriors; implants coated with 1% MF versus non-coated implants | 1% MF coated implant showed a bio-stimulatory effect on osseointegration |
| Seto H et al., (2008) [41] | Animal study on male Fischer rats | Fifteen male Fischer rats were experimentally included with periodontitis followed by a topical injection of 0.2 mg SM | SMV showed improved osteoblastic function |
| Pradeep AR et al., (2010) [35] | Human clinical trial | 60 patients with IBD; SRP+1.2 mg SMV versus SRP alone | SRP+SMV showed significant IBD fill |
| Martande SS et al., (2017) [36] | Human clinical trial | 96 patients; SRP+1.2% ATV, SRP+1.2% SMV and SRP plus placebo | ATV showed more significant defect depth reduction compared to SMV |
| Pradeep AR et al., (2015) [37] | Human clinical trial | 65 patients with IBD; SRP+RSV 1.2 mg and SRP plus placebo group | A significant reduction of IBD was seen with 1.2 mg RSV |
| Shoji K et al., (1995) [60] | Animal study on Wistar rats | 27 Wistar rats with experimentally induced periodontitis; 0.8, 1.6, or 3.2 moles/kg of Risedronate (test) and 0.9% NaCl | Doses of 1.6 and 3.2, moles/kg of Risedronate prevented alveolar bone resorption |
| Goes P et al., (2012) [61] | Animal study on Wistar rats | 36 Wistar; Groups of six animals received 0.9% saline or ALD (0.01; 0.05; 0.25 mg kg-1) | ALD reduced the bone-specific alkaline phosphatase and inflammatory infiltrate |
| Goya JA et al., (2006) [62] | Animal study on Wistar rats | 20 male Wistar rats; Experimental periodontitis (control group) and experimental periodontitis+topical olpadronate (test group) | Significant morphologic changes were seen in osteoclasts and the drug prevented bone loss |
| Tanna NK 2013 [64] | Human clinical trial | 125 patients with moderate to severe periodontitis; risedronate+SRP (test), SRP alone (control | The test group showed a significant increase in bone height |
| Sharma A and Pradeep AR, (2012) [65] | Human clinical trial | 66 patients with IBDs; 1% ALN+SRP, placebo gel+SRP | The test group showed improved bone fill |
| Arabacı T et al., (2015) [75] | Animal study on Sprague-Dawley rats | 24 rats with experimentally induced periodontitis; control, experimental periodontitis, and experimental periodontitis treated with MEL | Osteoclast activity was significantly lower in the MEL group |
| El-Sharkawy H et al., (2008) [79] | Human clinical trial | 74 periodontitis patients with primary insomnia; 10 mg oral MEL+SRP (test group), SRP alone | Test group showed improvements in clinical parameters and lower salivary TNFα |
| Tinto M et al., (2020) [80] Human clinical trial | 20 patients; SRP+1 mg MEL (test), SRP alone (control) | Improves clinical parameters were seen in the MEL group | |
MF: Metformin; SRP: Scaling and root planning; SM: Simvastatin; ATV: Atorvastatin; RSV: Rosuvastatin; ALD: Alendronate; MEL: Melatonin
Conclusion(s)
Drug repurposing is a cost-effective and expedited approach that brings effective therapies to patients faster compared to the lengthy traditional drug discovery and development processes. This approach helps counter the rising costs associated with drug development, which, in turn, reduces the financial burden on patients and ultimately lowers the overall cost of therapy. Furthermore, drug repurposing in periodontal treatment has the potential to reduce the reliance on antibiotics, which are frequently used as an adjunct to both surgical and non surgical periodontal therapies, thereby contributing to the mitigation of issues related to antibiotic resistance. While drug repurposing offers significant promise in this field, it is important to emphasise that rigorous research, clinical trials, and safety evaluations are essential before any repurposed drug can be recommended for widespread use.
[1]. Talevi A, Drug repositioning: Current approaches and their implications in the precision medicine eraExpert Review of Precision Medicine and Drug Development 2018 3(1):49-61. [Google Scholar]
[2]. Hodos RA, Kidd BA, Shameer K, Readhead BP, Dudley JT, In silico methods for drug repurposing and pharmacologyWiley Interdiscip Rev Syst Biol Med 2016 8(3):186-210. [Google Scholar]
[3]. Deotarse PP, Jain AS, Baile MB, Kolhe NS, Kulkarni AA, Drug repositioning: A reviewInternational Journal of Pharma Research & Review 2015 4(8):51-58. [Google Scholar]
[4]. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and ConditionsJ Periodontol 2018 89(Suppl 1):S173-82. [Google Scholar]
[5]. Ohlrich EJ, Cullinan MP, Seymour GJ, The immunopathogenesis of periodontal diseaseAust Dent J 2009 54(Suppl 1):S02-10. [Google Scholar]
[6]. Taubman MA, Valverde P, Han X, Kawai T, Immune response: The key to bone resorption in periodontal diseaseJ Periodontol 2005 76(11 Suppl):2033-41. [Google Scholar]
[7]. Sima C, Viniegra A, Glogauer M, Macrophage immunomodulation in chronic osteolytic diseases- The case of periodontitisJ Leukoc Biol 2019 105(3):473-87. [Google Scholar]
[8]. Slots J, Low-cost periodontal therapyPeriodontol 2000 2012 60(1):110-37. [Google Scholar]
[9]. Kirpichnikov D, McFarlane SI, Sowers JR, Metformin: An updateAnn Intern Med 2002 137(1):25-33. [Google Scholar]
[10]. Vigneri R, Goldfine ID, Role of metformin in treatment of diabetes mellitusDiabetes care 1987 10(1):118-22. [Google Scholar]
[11]. Rotermund C, Machetanz G, Fitzgerald JC, The therapeutic potential of metformin in neurodegenerative diseasesFront Endocrinol (Lausanne) 2018 9:400 [Google Scholar]
[12]. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G, Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver diseaseLiver Int 2010 30(5):750-58. [Google Scholar]
[13]. Zhen D, Chen Y, Tang X, Metformin reverses the deleterious effects of high glucose on osteoblast functionJ Diabetes Complications 2010 24(5):334-44. [Google Scholar]
[14]. Śmieszek A, Tomaszewski KA, Kornicka K, Marycz K, Metformin promotes osteogenic differentiation of adipose-derived stromal cells and exerts pro-osteogenic effect stimulating bone regenerationJ Clin Med 2018 7(12):482 [Google Scholar]
[15]. Kang W, Wang T, Hu Z, Liu F, Sun Y, Ge S, Metformin inhibits P. gingivalis Lipopolysaccharide (LPS)-influenced Inflammatory Response in Human Gingival Fibroblasts via Regulating Activating Transcription Factor-3 (ATF3) ExpressionJ Periodontol 2017 88(10):e169-78. [Google Scholar]
[16]. Xu W, Tan W, Li C, Wu K, Zeng X, Xiao L, Metformin-loaded β-TCP/CTS/SBA-15 composite scaffolds promote alveolar bone regeneration in a rat model of periodontitisJ Mater Sci Mater Med 2021 32(12):01-03. [Google Scholar]
[17]. Neves VC, Satie Okajima L, Elbahtety E, Joseph S, Daly J, Menon A, Repurposing Metformin for periodontal disease management as a form of oral-systemic preventive medicineJ Transl Med 2023 21(1):655 [Google Scholar]
[18]. Pradeep AR, Rao NS, Naik SB, Kumari M, Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: A randomized controlled clinical trialJ Periodontol 2013 84(2):212-20. [Google Scholar]
[19]. Pradeep AR, Patnaik K, Nagpal K, Karvekar S, Ramamurthy BL, Naik SB, Efficacy of locally-delivered 1% metformin gel in the treatment of intrabony defects in patients with chronic periodontitis: A randomized, controlled clinical trialJ Investig Clin Dent 2016 7(3):239-45. [Google Scholar]
[20]. Swami RK, Kolte AP, Kolte RA, Clinico-radiographic comparative evaluation of 1% metformin gel plus platelet-rich fibrin over platelet-rich fibrin alone in the treatment of Grade II furcation defects: A randomized controlled double-blind clinical trialJ Periodontol 2022 93(5):644-55. [Google Scholar]
[21]. Sharma H, Sharma A, Gupta S, Mowar A, Kusum C, Evaluation of the effect of 1% Metformin Gel coating on the osseointegration achieved around dental implants using bone mineral density and resonance frequency analysis- A clinical and three-dimensional radiographic studyInternational Journal of Pharmaceutical Research 2021 13(1):393-401. [Google Scholar]
[22]. Aljofi FE, Alesawy A, Alzaben B, Alshaikh M, Alotaibi N, Aldulaijan HA, Impact of metformin on periodontal and peri-implant soft and hard tissueInt J Environ Res Public Health 2023 20(2):1095 [Google Scholar]
[23]. Gelissen IC, McLachlan AJ, The pharmacogenomics of statinsPharmacol Res 2014 88:99-106. [Google Scholar]
[24]. Liao JK, Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterolAm J Cardiol 2005 96(5A):24-33. [Google Scholar]
[25]. Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancerBreast Cancer Res Treat 2010 119(1):137-44.19728082 [Google Scholar] [PubMed]
[26]. Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthaseProc Natl Acad Sci USA 1998 95(15):8880-85. [Google Scholar]
[27]. Parsaik AK, Singh B, Hassan MM, Singh K, Mascarenhas SS, Williams MD, Statins use and risk of depression: A systematic review and meta-analysisJ Affect Disord 2014 160:62-67. [Google Scholar]
[28]. Kiener PA, Davis PM, Murray JL, Youssef S, Rankin BM, Kowala M, Stimulation of inflammatory responses invitro and invivo by lipophilic HMG-CoA reductase inhibitorsInt Immunopharmacol 2001 1(1):105-18. [Google Scholar]
[29]. Zhu X, Parks JS, New roles of HDL in inflammation and hematopoiesisAnnu Rev Nutr 2012 32:161-82. [Google Scholar]
[30]. Parolina de Carvalho RD, de Andrade Moreno J, Roque SM, Chan DCH, Torrez WB, Stipp RN, Statins and oral biofilm: Simvastatin as a promising drug to control periodontal dysbiosisOral Dis 2024 30(2):669-80. [Google Scholar]
[31]. Sakoda K, Yamamoto M, Negishi Y, Liao JK, Node K, Izumi Y, Simvastatin decreases IL-6 and IL-8 production in epithelial cellsJ Dent Res 2006 85(6):520-23. [Google Scholar]
[32]. Liu C, Wu Z, Sun HC, The effect of simvastatin on mRNA expression of transforming growth factor-β1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socketInt J Oral Sci 2009 1(2):90-98. [Google Scholar]
[33]. Poston CJ, Pierce TC, Li Y, Brinson CW, Lu Z, Lauer AW, Statin intake is associated with MMP-1 level in gingival crevicular fluid of patients with periodontitisOral Dis 2016 22(5):438-44. [Google Scholar]
[34]. Park A, Barrera-Ramirez J, Ranasinghe I, Pilon S, Sy R, Fergusson D, Use of statins to augment progenitor cell function in preclinical and clinical studies of regenerative therapy: A systematic reviewStem Cell Rev Rep 2016 12(3):327-39. [Google Scholar]
[35]. Pradeep AR, Thorat MS, Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: A randomized clinical trialJ Periodontol 2010 81(2):214-22. [Google Scholar]
[36]. Martande SS, Kumari M, Pradeep AR, Singh SP, Suke DK, Comparative evaluation of efficacy of subgingivally delivered 1.2% Atorvastatin and 1.2% Simvastatin in the treatment of intrabony defects in chronic periodontitis: A randomized controlled trialJ Dent Res Dent Clin Dent Prospects 2017 11(1):18-25. [Google Scholar]
[37]. Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Guruprasad CN, Kumaraswamy KM, Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: A randomized, placebo-controlled clinical trialJ Periodontol 2015 86(6):738-45. [Google Scholar]
[38]. Emani S, Gunjiganur GV, Mehta DS, Determination of the antibacterial activity of simvastatin against periodontal pathogens, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An invitro studyContemp Clin Dent 2014 5(3):377-82. [Google Scholar]
[39]. Das S, Pudakalkatti PS, Vaz A, Kour P, Padmanabhan S, Determination of the antibacterial activity of atorvastatin against periodontal pathogens, aggregatibacter actinomycetemcomitans and porphyromonas gingivalis: An in vitro studyJ Interdiscip Dent 2020 10(1):03-08. [Google Scholar]
[40]. Liu S, Bertl K, Sun H, Liu ZH, Andrukhov O, Rausch-Fan X, Effect of simvastatin on the osteogenetic behavior of alveolar osteoblasts and periodontal ligament cellsHum Cell 2012 25(2):29-35. [Google Scholar]
[41]. Seto H, Ohba H, Tokunaga K, Hama H, Horibe M, Nagata T, Topical administration of simvastatin recovers alveolar bone loss in ratsJ Periodontal Res 2008 43(3):261-67. [Google Scholar]
[42]. Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A, Statins in nonsurgical and surgical periodontal therapy. A systematic review and meta-analysis of preclinical invivo trialsJ Periodontal Res 2018 53(3):267-87. [Google Scholar]
[43]. Kellesarian SV, Al Amri MD, Al-Kheraif AA, Ghanem A, Malmstrom H, Javed F, Efficacy of local and systemic statin delivery on the osseointegration of implants: A systematic reviewInt J Oral Maxillofac Implants 2017 32(3):497-506. [Google Scholar]
[44]. Blomen LJ, History of the bisphosphonates: Discovery and history of the non-medical uses of bisphosphonates. Chapter 7. In: Bijvoet O, Fleisch HA, Canfield RE, Russell RGG, editorsBisphosphonates on bone 1995 Elsevier Science B.V.:111-24. [Google Scholar]
[45]. Fleisch H, Reszka A, Rodan G, Rogers M, Bisphosphonates: Mechanisms of actionPrinciples of Bone Biology 2002 :1361-XLIII. [Google Scholar]
[46]. Fleisch H, Graham R, Russell G, Francis MD, Diphosphonates inhibit hydroxyapatite dissolution invitro and bone resorption in tissue culture and invivoScience 1969 165(3899):1262-64. [Google Scholar]
[47]. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Ten years’ experience with alendronate for osteoporosis in postmenopausal womenN Engl J Med 2004 350(12):1189-99. [Google Scholar]
[48]. Fraser WD, Stamp TC, Creek RA, Sawyer JP, Picot C, A double-blind, multicentre, placebo-controlled study of tiludronate in Paget’s disease of bonePostgrad Med J 1997 73(862):496-502. [Google Scholar]
[49]. Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Zoledronic acid is superior to pamidronate in the treatment of hypercalcaemia of malignancy: A pooled analysis of two randomized, controlled clinical trialsJ Clin Oncol 2001 19(2):558-67. [Google Scholar]
[50]. Marshall JK, The gastrointestinal tolerability and safety of oral bisphosphonatesExpert Opin Drug Saf 2002 1(1):71-78. [Google Scholar]
[51]. Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, Toxic acute tubular necrosis following treatment with zoledronate (Zometa)Kidney Int 2003 64(1):281-89. [Google Scholar]
[52]. Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factorsJ Clin Oncol 2005 23(34):8580-87. [Google Scholar]
[53]. Ficarra G, Beninati F, Rubino I, Vannucchi A, Longo G, Tonelli P, Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatmentJ Clin Periodontol 2005 32(11):1123-28. [Google Scholar]
[54]. Jobke B, Milovanovic P, Amling M, Busse B, Bisphosphonate-osteoclasts: Changes in osteoclast morphology and function induced by antiresorptive nitrogen-containing bisphosphonate treatment in osteoporosis patientsBone 2014 59:37-43. [Google Scholar]
[55]. Nakaya H, Osawa G, Iwasaki N, Cochran DL, Kamoi K, Oates TW, Effects of bisphosphonate on matrix metalloproteinase enzymes in human periodontal ligament cellsJ Periodontol 2000 71(7):1158-66. [Google Scholar]
[56]. Giannobile WV, Host-response therapeutics for periodontal diseasesJ Periodontol 2008 79(Suppl 8):1592-600. [Google Scholar]
[57]. Açil Y, Möller B, Niehoff P, Rachko K, Gassling V, Wiltfang J, The cytotoxic effects of three different bisphosphonates in-vitro on human gingival fibroblasts, osteoblasts and osteogenic sarcoma cellsJ Craniomaxillofac Surg 2012 40(8):e229-35. [Google Scholar]
[58]. Açil Y, Arndt ML, Gülses A, Wieker H, Naujokat H, Ayna M, Cytotoxic and inflammatory effects of alendronate and zolendronate on human osteoblasts, gingival fibroblasts and osteosarcoma cellsJ Craniomaxillofac Surg 2018 46(4):538-46. [Google Scholar]
[59]. Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA, The effects of bisphosphonates on osteoblasts invitroOral Surg Oral Med Oral Pathol Oral Radiol Endod 2008 106(1):05-13. [Google Scholar]
[60]. Shoji K, Horiuchi H, Shinoda H, Inhibitory effect of a bisphosphonate (risedronate) on experimental periodontities in ratsJ Periodontal Res 1995 30(4):277-84. [Google Scholar]
[61]. Goes P, Melo IM, Dutra CS, Lima AP, Lima V, Effect of alendronate on bone-specific alkaline phosphatase on periodontal bone loss in ratsArch Oral Biol 2012 57(11):1537-44. [Google Scholar]
[62]. Goya JA, Paez HA, Mandalunis PM, Effect of topical administration of monosodium olpadronate on experimental periodontitis in ratsJ Periodontol 2006 77(1):01-06. [Google Scholar]
[63]. Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, Bisphosphonate therapy improves the outcome of conventional periodontal treatment: Results of a 12-month, randomized, placebo-controlled studyJ Periodontol 2005 76(7):1113-22. [Google Scholar]
[64]. Tanna NK, Jeffcoat MK, Actonel® (risedronate) therapy for the maintenance of alveolar bone in adult chronic periodontitisHealth 2013 5(7B):12-17. [Google Scholar]
[65]. Sharma A, Pradeep AR, Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: A randomized, controlled clinical trialJ Periodontol 2012 83(1):11-18. [Google Scholar]
[66]. Dutra BC, Oliveira AM, Oliveira PA, Manzi FR, Cortelli SC, Cota LO, Effect of 1% sodium alendronate in the non-surgical treatment of periodontal intraosseous defects: A 6-month clinical trialJ Appl Oral Sci 2017 25(3):310-17. [Google Scholar]
[67]. Alwithanani N, Role of bisphosphonates in periodontal diseases: Systematic reviewJ Pharm Bioallied Sci 2023 15(Suppl 1):S46-53. [Google Scholar]
[68]. Akram Z, Abduljabbar T, Kellesarian SV, Abu Hassan MI, Javed F, Vohra F, Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: A systematic reviewBr J Clin Pharmacol 2017 83(3):444-54. [Google Scholar]
[69]. Bobyn DJ, Thompson R, Lim L, Pura JA, Bobyn K, Tanzer M, Local alendronic acid elution increases net periimplant bone formation: A micro-CT analysisClin Orthop Relat Res 2014 472(2):687-94. [Google Scholar]
[70]. Mccord CP, Allen FP, Evidences associating pineal gland function with alterations in pigmentationJournal of Experimental Zoology 1917 23(1):207-24. [Google Scholar]
[71]. Samanta S, Physiological and pharmacological perspectives of melatoninArch Physiol Biochem 2022 128(5):1346-67. [Google Scholar]
[72]. Carpentieri A, De Barboza GD, Areco V, López MP, De Talamoni NT, New perspectives in melatonin usesPharmacol Res 2012 65(4):437-44. [Google Scholar]
[73]. Gonde NP, Rathod SR, Kolte AP, Comparative evaluation of 1% melatonin gel in the treatment of intrabony defect: A randomized controlled clinical trialJ Periodontol 2022 93(12):1878-88. [Google Scholar]
[74]. Meenakshi SS, Malaiappan S, Role of melatonin in periodontal disease- A systematic reviewIndian J Dent Res 2020 31(4):593-600. [Google Scholar]
[75]. Arabacı T, Kermen E, Özkanlar S, Köse O, Kara A, Kızıldağ A, Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: A biochemical and immunohistochemical studyJ Periodontol 2015 86(7):874-81. [Google Scholar]
[76]. Cutando A, Montero J, Gómez-de Diego R, Ferrera MJ, Lopez-Valverde A, Effect of topical application of melatonin on serum levels of C-reactive protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α) in patients with type 1 or type 2 diabetes and periodontal diseaseJ Clin Exp Dent 2015 7(5):e628-33. [Google Scholar]
[77]. Cutando A, López-Valverde A, Gómez-de-Diego R, Arias-Santiago S, de Vicente-Jiménez J, Effect of gingival application of melatonin on alkaline and acid phosphatase, osteopontin and osteocalcin in patients with diabetes and periodontal diseaseMed Oral Patol Oral Cir Bucal 2013 18(4):e657-63. [Google Scholar]
[78]. Cutando A, López-Valverde A, de Diego RG, de Vicente J, Reiter R, Herrero Fernández M, Effect of topical application of melatonin to the gingiva on salivary osteoprotegerin, RANKL and melatonin levels in patients with diabetes and periodontal diseaseOdontology 2014 102(2):290-96. [Google Scholar]
[79]. El-Sharkawy H, Elmeadawy S, Elshinnawi U, Anees M, Is dietary melatonin supplementation a viable adjunctive therapy for chronic periodontitis?-A randomized controlled clinical trialJ Periodontal Res 2019 54(2):190-97. [Google Scholar]
[80]. Tinto M, Sartori M, Pizzi I, Verga A, Longoni S, Melatonin as host modulating agent supporting nonsurgical periodontal therapy in patients affected by untreated severe periodontitis: A preliminary randomized, triple-blind, placebo-controlled studyJ Periodontal Res 2020 55(1):61-67. [Google Scholar]
[81]. Gómez-Moreno G, Aguilar-Salvatierra A, Boquete-Castro A, Guardia J, Piattelli A, Perrotti V, Outcomes of topical applications of melatonin in implant dentistry: A systematic reviewImplant Dent 2015 24(1):25-30. [Google Scholar]