The EMB stands out as the most prevalent contributor to optic nerve toxicity. According to the World Health Organisation (WHO), with an annual global TB incidence of around 9.2 million cases, of which approximately 55% resort to EMB for treatment its role becomes pivotal [1]. Taking a conservative estimate that 2% of these cases will suffer significant and irreversible visual impairment, the yearly tally of this profound iatrogenic complication is projected at 100,000 cases [2]. The use of EMB has been linked to potential manifestations of ocular toxicity, specifically deteriorating visual acuity or impairment in red-green colour discrimination [3]. India, bearing a substantial burden in the global TB scenario, accounts for a noteworthy one-fifth of the total cases. In response to this gigantic problem, the country has implemented a Revised National Tuberculosis Control Programme (RNTCP) across its expanse. The early detection of ocular symptoms is important to prevent unnecessary delay in diagnosis and probable irreversible visual loss [4]. Since its first use in the treatment of TB, the potential for toxic optic neuropathy secondary to EMB has been well recognised. Early animal experiments showed that EMB causes lesions in the optic chiasm and optic nerves [5]. The objective assessment of optic nerve and intracranial visual pathway function is effectively facilitated by electrophysiological investigations [6]. VEPs refer to electrophysiological signals elicited by visual stimuli and derived from the electroencephalographic activity within the visual cortex. These signals are captured through scalp recordings, providing insights into the neural responses associated with visual processing [7]. The typical pattern-reversal VEP waveform comprises distinct peaks, including N75, P100 and N145 [7]. P100, a prominent and relatively stable peak, demonstrates minimal variation between subjects, limited interocular differences within individuals and consistency across repeated measurements over time [7]. The utilisation of VEP pattern reversal is recommended due to its minimal intra- and inter-individual variability compared to non pattern stimuli. This method, employing a checkerboard pattern, enhances the detection of subtle visual pathway abnormalities with superior accuracy compared to flash VEP [8].
It’s noteworthy that physical parameters also impact VEP outcomes. Research indicates that VEP waves are notably longer in males compared to females and the amplitude of the P100 wave tends to be higher in females for both eyes in contrast to males [9]. If patients exhibit co-morbidities that heighten their susceptibility to EMB toxicity, such as renal impairment or diabetes, it might necessitate dose adjustments or monitoring. Seeking advice from specialists, including ophthalmologists, becomes crucial in these cases. Conversely, when treating fully sensitive TB, omitting EMB may be considered [10]. Despite this, EMB remains in use due to its effective anti-mycobacterial action coupled with a relatively favourable systemic safety profile. Fixed drug combinations wherein a single tablet contains a fixed strength of isoniazid, rifampicin, pyrazinamide and EMB are being used under the RNTCP 2016 guidelines. The number of tablets prescribed is based on the weight [11]. Studies have indicated that individuals with concurrent hypertension, diabetes and renal conditions face an elevated risk of EMB Optic Neuropathy (EON) [12]. The research postulates that age acts as an independent risk factor for EON, attributing this to the presumed decline in renal function associated with aging. While it is recognised that renal tubular function diminishes with age, the specific impact of aging on EMB clearance remains an aspect yet to be fully elucidated [12]. Interestingly, smoking is listed as a potential risk factor for EON, although prior studies have not consistently associated smoking with this condition [13].
In the absence of renal impairment, the standard daily dosage for EMB during the entire anti- TB treatment course is recommended at 15 mg/kg per day [14]. Nevertheless, in specific scenarios like severe cavitary TB, drug-resistant TB, or cases requiring retreatment, the consideration of an elevated dosage of 25 mg/kg per day is warranted. It’s crucial to note that this higher dose should not be administered for a duration exceeding two months. For individuals classified as obese, calculations should be based on their ideal body weight [14]. Many authorities widely acknowledge that the toxicity associated with this particular medication is considered reversible upon prompt discontinuation of the drug. The prevailing belief among experts is that taking swift action to cease the medication can effectively bring about the reversal of the toxicity, establishing it as a commonly perceived and manageable reversible condition [15]. Based on one Korean study, the incidence of EON in Koreans is estimated to be <2%. However, visual function after discontinuation of EMB is reversible in only a minority of patients and does not occur if optic disc pallor is present. Renal dysfunction and the daily dose of EMB, but not the duration of EMB treatment, seem to be related to the development of EON [16]. The WHO recommended daily dosage of EMB for TB is 15-20 mg/kg/day and at the current dose, the incidence of EON is 1%-3% [17]. EMB toxicity has been recognised as being dose-related, with a reported incidence of 18% in patients receiving >35 mg/kg/day, 5-6% with 25 mg/kg/day and <1% with 15 mg/kg/day of EMB for more than two months [18]. The aim of the study was to assess the use of VEP to identify EON by detecting subtle changes in visual function. The objective of the study was to assess the relation of VEP changes in EON with duration and dose of treatment. The alternative hypothesis was that the VEP can accurately diagnose EON, showing significant changes in amplitude compared to control groups or baseline measurements. Null hypothesis was that there is no significant difference in VEP responses between patients with EON and control groups, indicating that VEP is not an effective predictor or diagnostic tool for this condition.
Materials and Methods
The cross-sectional study was conducted at the Department of Physiology, B.J. Medical College, Ahmedabad, Gujarat, India, from August 2023 to December 2023. VEP was recorded using a PC-based, 2-channel RMS NCV EMG MARK II machine manufactured by Recorders and Medicare Systems Pvt. Ltd., along with standard silver-silver chloride disc electrodes. The present study obtained ethical clearance from the Institutional Ethical Committee, BJ Medical College, Ahmedabad, with approval letter no. EC/Approval/99/2023 dated 30-10-2023. VEP recordings were performed one at a time, regardless of the treatment period and consent was obtained from all participants.
Inclusion criteria for cases:
Age 18 ≥years,
A diagnosis of TB requires a combination of ATT, including EMB at a dose of 15-25 mg/kg daily.
Subjects with visual acuity of 6/60, finger counting present (assessed by an ophthalmologist).
Inclusion criteria for controls:
Age 18 ≥years,
Subjects with corrected visual acuity.
Exclusion criteria for cases:
Patients with any known pathology of the optic nerve or retina were excluded from the study.
Exclusion criteria for controls:
Having any visual symptoms or any systemic disease that affects the eye.
Non cooperative.
An informed consent form was obtained from all individual participants included in the study.
Study Procedure
The study included patients aged 19-70 years who were receiving EMB as part of their TB treatment, while others were considered normal. There were a total of 31 subjects, with 15 being TB patients and 16 being normal subjects. The participants consisted of 16 men and 15 women. All patients were undergoing various combinations of Anti-Tuberculous Treatment (ATT), including EMB and had been on this treatment for an average of 6±4 months before discontinuation due to suspected EON. The dose of EMB administered was 15-20 mg/kg daily.
Fixed drug combinations, where a single tablet contains a fixed strength of isoniazid, rifampicin, pyrazinamide and EMB, were used according to the RNTCP 2016 guidelines. The number of tablets prescribed is determined by the weight band of the patient [Table/Fig-1], which may provide some protection against ocular toxicity [11].
Dose of Ethambutol (EMB) in adult patients based on weight scale.
| Weight category | Number of tablets |
|---|
| Intensive phase HRZE 75/150/400/275 (mg) | Continuation phase HRE 75/150/275 (mg) |
| 25-39 kg | 2 | 2 |
| 40-54 kg | 3 | 3 |
| 55-69 kg | 4 | 4 |
| ≥70 kg | 5 | 5 |
H Isoniazid; R Rifampicin; Z Pyrazinamide; E Ethambutol
A one-channel montage was used to record the VEP. Skin electrodes such as sintered silver-silver chloride, standard silver-silver chloride, or gold cup electrodes are recommended for recording VEPs [Table/Fig-2]. The skin should be prepared by cleaning and a suitable paste or gel should be used to ensure a good, stable electrical connection. The scalp electrodes were placed according to the International 10/20 system. The anterior and posterior midline measurements are based on the distance between the nasion and the inion over the vertex. The active electrode was placed at Oz, which is the highest point of the occiput and lies over the visual cortex. The reference and ground electrodes were placed at Fz (forehead) and Cz (vertex), respectively.
Procedure of VEP (Showing machine and electrodes).

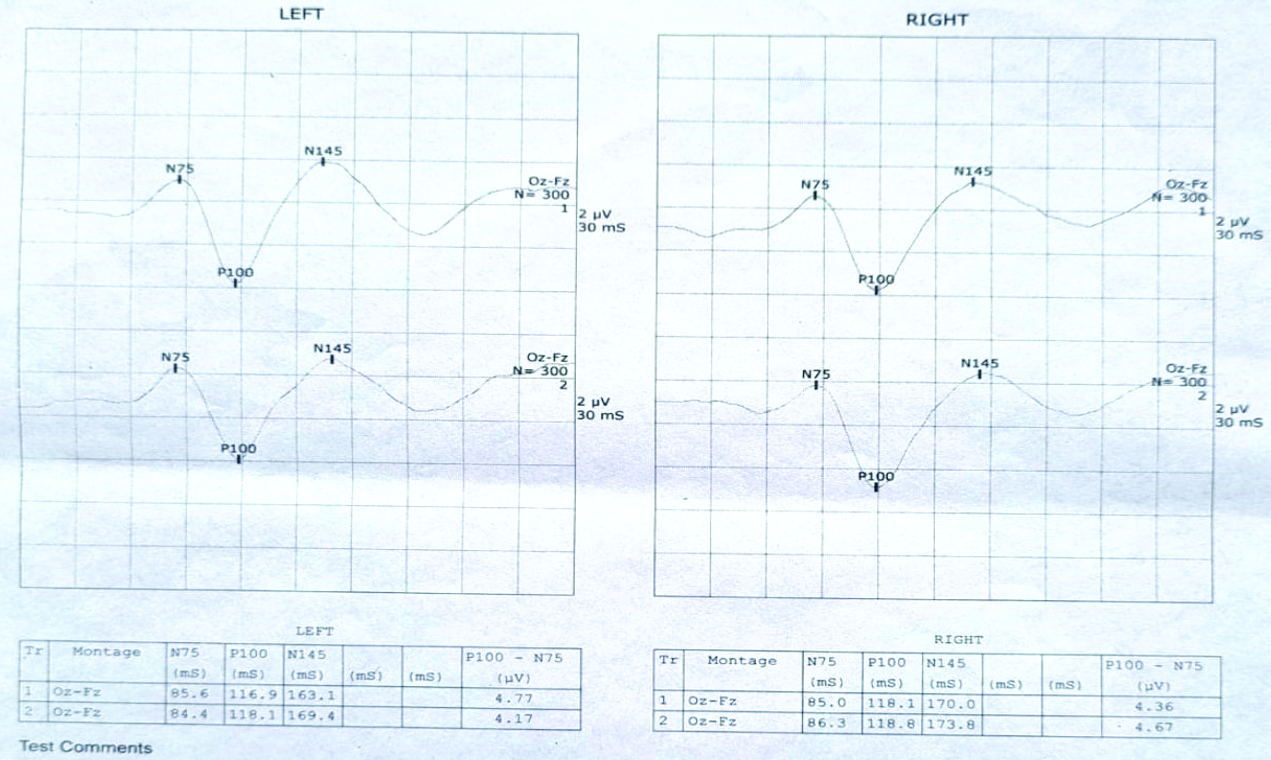
Pattern-reversal VEPs (PVEP) was utilised by us. N75, P100 and N145 are three of the different peaks that make up the normal pattern-reversal VEP waveform [Table/Fig-3]. N and P stand for negative and positive polarity, respectively. After pattern reversal, peak latencies are reported in milliseconds. The occiput is the location of peaks N75, P100 and N145 [19]. P100 is a noticeable and somewhat stable peak that shows little fluctuation across subjects, little interocular variance within individuals and stability across time in many assessments [7]. Because VEP pattern reversal has less intra- and inter-individual variability than non pattern stimuli, it is advised to use it. Compared to flash VEP, this approach, which uses a checkerboard pattern, improves the detection of small visual pathway anomalies with higher accuracy [8].
VEP record of normal subject.
A quiet environment with minimal light was used for the recording. The participant was instructed to concentrate on the screen’s center while seated at a constant distance of 100 cm from the screen. Separate monocular stimulation was applied to each eye. A total of 300 responses were averaged across a 250-500 ms sweep duration. The amplification range that was used was 20,000 to 100,000. It was maintained that the electrode impedance was less than 5 KΩ [7].
Stimulus used:
Responses to patterned visual stimuli show significantly less variation within and between individuals compared to responses to unpatterned stimuli.
PVEP testing is more sensitive and accurate at detecting minor abnormalities in the visual pathway than FVEP testing.
Checkerboard pattern reversal is widely preferred as a pattern stimulus due to its simplicity and reliability.
Unpatterned stimuli are typically used for patients who have difficulty fixating on or paying attention to the stimulus.
Additionally, unpatterned stimuli are valuable for studying steady-state VEPs.
When using pattern stimulation, adjusting parameters like check size, field size and field location allows targeted testing of specific visual pathway segments.
Checkerboard pattern stimuli were used in all subjects.
Statistical Analysis
The mean and standard deviation of VEP wave latencies and amplitudes were computed. Statistical analysis was performed using Student’s unpaired t-test to compare the results between both groups and p-values were derived.
Results
In the study population of 31, 16 (52%) were men and 15 (48%) were women. There were a total of 31 subjects, of which 15 were TB patients and 16 were normal subjects. Most of the patients in the present study had Lung TB (53.3%). Only 3 (20%) patients had a duration of more than eight months for ATT [Table/Fig-4].
Types of Tuberculosis (TB) with duration of ATT and visual symptoms (Reduced visual acuity ≤6/60, FC present).
| Cases | Drug h/o (in months) | TB type | Visual symptoms (in days) | EMB dose (mg/kg/day) |
|---|
| 1 | 9 | TB spine | 15 | 19 |
| 2 | 2 | TB lung | 19 | 16 |
| 3 | 12 | TB meningitis | 60 | 20 |
| s | 4 | TB lung | 14 | 18 |
| 5 | 6 | MDR TB | 75 | 18 |
| 6 | 2.5 | TB lung | 20 | 20 |
| 7 | 3.5 | TB lung | 18 | 17 |
| 8 | 18 | TB abdominal MDR | 30 | 16 |
| 9 | 5.5 | TB lung | 12 | 17 |
| 10 | 5 | TB spine | 35 | 20 |
| 11 | 8 | TB abdominal | 28 | 17 |
| 12 | 7.5 | MDR TB | 32 | 19 |
| 13 | 5 | TB lung | 25 | 18 |
| 14 | 4.8 | TB lung | 27 | 16 |
| 15 | 3 | TB lung | 35 | 20 |
MDR: Multidrug resistant; FC: Finger counting
The duration of EMB therapy was 6.38±4.034 months and the average EMB dose (mg/kg/day) was 18±1.48. Visual acuity was <6/60 and finger counting was present (assessed by an ophthalmologist), with a duration of visual symptoms of 34.4±22.17 days.
There were 15 patients with TB with a mean age of 40±17.4 years and a mean weight of 55.86±11.21 kg who complained of visual impairment for around 12-75 days. A total of 16 normal subjects with a mean age of 31.12±12.54 years and a mean weight of 64.37 kg were taken as the control group [Table/Fig-5].
Mean age and weight distribution in cases.
| Variables | Cases | Controls |
|---|
| Age (years) | 40±17.4 | 31.12±12.54 |
| Weight (kg) | 55.86±11.21 | 64.37±11.71 |
According to [Table/Fig-6], the P100 mean amplitude of the left eyes in cases is 7.3±0.45 mV, while in controls it is 3.725±0.624 mV. The P100 mean amplitude of the right eyes in cases is 0.7±0.46 mV, whereas in controls it is 3.62±0.673 mV. The two-tailed p-value is less than 0.0001 for both sides, indicating an extremely statistically significant difference. Therefore, it suggests that VEP P100 amplitude decreased considerably in EON.
Amplitude of P100 in left and right eyes.
| P100 amplitude (in millivolt) | Cases | Controls | p-value |
|---|
| Left eyes | 0.7293±0.4514 | 3.7438±0.6149 | 0.0001 |
| Right eyes | 0.7±0.46 | 3.62±0.673 | 0.0001 |
According to [Table/Fig-7], the P100 mean latency in the left eyes of controls is 112.94±3.51 ms, while in cases it is 105.93±11.36 ms. The P100 mean latency in the right eyes of controls is 114.018±3.398 ms, compared to 106.73±12.068 ms in cases. The two-tailed p-value is 0.0273 for the left eyes and 0.0275 for the right eyes in [Table/Fig-6], indicating a very statistically significant difference.
Latency of P100 in left and right eyes.
| P100 latency (in milliseconds) | Cases | Controls | p-value |
|---|
| Left eyes | 105.93±11.36 | 112.93±3.91 | 0.0273 |
| Right eyes | 106.73±12.068 | 114.018±3.398 | 0.0275 |
In [Table/Fig-8], the N75 and N145 latencies of cases are slightly prolonged compared to controls.
Latencies of N75 and N145.
| Latency (in milliseconds) | Controls | Cases |
|---|
| N75 left | 73.18±18.06 | 81.44±5.44 |
| N75 right | 76.73±6.48 | 81.96±6.16 |
| N145 left | 149±16.45 | 158.18±8.53 |
| N145 right | 151.93±20 | 161.12±9.76 |
Discussion
The present study assessed knowledge and attitudes regarding VEP’s utility as a diagnostic tool for EMB-induced optic neuropathy by detecting significant amplitude changes in visual function. VEP emerges as a valuable monitoring tool for patients undergoing EMB therapy.
The visual impairment associated with EMB is suggested to be mediated through an excitotoxic pathway. This involves rendering ganglion cells sensitive to levels of extracellular glutamate that would normally be tolerated. EMB is known to disrupt mitochondrial function and its toxicity may be associated with decreased Adenosine Triphosphatase (ATPase) activity and disturbances in mitochondrial energy homeostasis. The review suggests that the use of glutamate antagonists could prove beneficial in limiting the side-effects observed with EMB [20].
The EMB toxicity has been identified as being dose-related in some cases. A reported incidence of 18% in patients receiving >35 mg/kg/day, 5-6% with 25 mg/kg/day and <1% with 15 mg/kg/day of EMB for more than two months [18]. In this study, the dose was 15-20 mg/kg daily, but subjects still showed EON development. This suggests that dose may not be an important factor in terms of prevention.
In one study, it was demonstrated that the duration of treatment and the interval from visual loss to stopping EMB are important markers for presenting visual acuity. Interestingly, patients with EON who were on medication for longer periods (≥8 months) presented with better vision compared to those who were on it for less than eight months [15]. This finding may suggest the presence of some protective property or trait in certain patients that prevents more serious optic neuropathy from developing. More importantly, it indicates that patients who are going to experience more severe visual decline are likely to do so earlier in the treatment (within the first eight months). Therefore, patients should be monitored closely during the first eight months of treatment with EMB [15]. In the present study, we also included patients with a treatment duration of an average of 6±4 months. Therefore, patients should be monitored early after starting treatment. In this study, only three patients had a duration longer than eight months. So, according to the present study, other factors may be more important than duration and should be evaluated in future studies.
In optic neuropathy, other than visual acuity, a visual field defect, decreased colour vision and the presence of a Relative Afferent Papillary Defect (RAPD) are seen. Optical Coherence Tomography (OCT) is a new technology that uses low-coherence light to penetrate tissue and a camera to analyse the reflected image. By performing circular scans around the optic nerve head, the peripapillary nerve fiber layer can be analysed. This has been useful in the follow-up of patients with optic neuritis [21]. In this study, the authors didn’t follow-up on patients with optic neuritis. In a past study, 17 (65.4%) eyes of nine patients showed delayed P100 VEP latency (127.70±18.46 ms in pattern VEP and 140.90±12.78 ms in flash VEP). In 2 patients (4 eyes), there was no response in pattern VEP, whereas P100 latency was delayed in flash VEP and in one patient (two eyes), the amplitude of the flash VEP was extinguished, whereas there was P100 delay in pattern VEP [16]. In the present study, we conducted pattern VEP in all patients, which showed that latency is shorter in cases compared to controls.
Due to the potential (though rare) toxic effects of EMB on the eyes, it is recommended to test visual acuity using a Snellen chart before prescribing the medication. The drug should only be prescribed to patients who have adequate visual acuity and are capable of recognising and reporting any visual symptoms or changes. Documentation should include that the patient has been instructed to discontinue the medication immediately if such symptoms occur and to notify their physician. The general practitioner should also be informed [22]. Therefore, prior visual assessment helps to prevent this optic neuropathy.
Here, an alternative hypothesis is accepted. VEP can accurately diagnose EON, showing a significant decrease in amplitude compared to control groups or baseline measurements. Therefore, the null hypothesis is rejected.
The VEP can aid in the diagnosis of EON, especially in cases where clinical symptoms are ambiguous or when other diagnostic tests, like visual field testing or fundoscopic examination, are inconclusive. Abnormal VEP findings consistent with optic nerve dysfunction can support the diagnosis of EON. Utilising VEP for early detection can facilitate timely intervention, potentially preventing further optic nerve damage and preserving vision.
Patients who are planning to undergo EMB treatment should be explained in detail about the possibility of vision loss, the need for periodic screening and follow-up with an ophthalmologist, including vigilance to identify any visual disturbance early. Physicians should be sensitised about the risk of EMB toxicity and the high incidence of subclinical damage. Proper dose adjustment of EMB decreases the incidence of vision loss. Also, early detection of risk factors for EON helps reduce incidence.
Limitation(s)
The study did not include TB patients without visual symptoms on EMB. Therefore, early changes in VEP were not noted. No follow-up was done with patients regarding VEP, so no follow-up changes could be seen after the discontinuation of EMB.
Conclusion(s)
The present research shows a significant reduction in P100 amplitude in EON compared to controls. Integrating VEP into routine ophthalmologic assessments for EMB-receiving patients could enhance TB management with proper dose adjustment while decreasing the associated risk of vision loss. Research aimed at developing neuroprotective strategies for preventing or mitigating EMB-induced optic nerve damage can benefit from incorporating VEP as an outcome measure to evaluate treatment efficacy. Integration of VEP with other diagnostic modalities, such as OCT and Magnetic Resonance Imaging (MRI), can provide a comprehensive assessment of optic nerve structure and function in EON, offering valuable insights into disease pathophysiology.
MDR: Multidrug resistant; FC: Finger counting