Breast carcinomas are the most heterogenous group of neoplasms in terms of their clinical, pathological and molecular variability [1] and one of the most common malignancies in current era [2]. While some have indolent course, few like TNBCs are notorious for aggressive clinical outcomes [3]. Different biomarkers and molecular profiling are being attempted to prognosticate outcomes of different breast carcinoma patients and also for implementation of novels molecular targets [4]. The Ki-67 is one such biomarker that utilises proliferation potential of breast carcinoma as one of the main indicators of aggressiveness, which lacks a standardised cut-off for grading the neoplasm [5,6].
Minichromosome Maintenance 2 is a part of complex MCM2-7 that belongs to the family of minichromosome maintenance proteins and is regulated by a group of transcription factors also involved in breast carcinomas [7]. The MCM2 has both cell proliferation associated domain and apoptosis enhancing domain, with important role in DNA damage response, replication and genomic stability [8]. Furthermore, its role in DNA repair and replication initiation has been utilised time and again as novel therapeutic targets in colon cancers and lung cancers [9,10]. Here, it has been evaluated as a proliferation marker with respect to different aspects of breast carcinogenesis.
The objective was to study the expression of MCM2 and Ki-67 in breast carcinomas by Immunohistochemistry (IHC). The grades of MCM2 expression were compared with histologic grade, type, staging, nodal status, molecular subtypes and Ki-67 expression. Any correlation between grades of MCM2 and Ki-67 expression was also evaluated.
Materials and Methods
This cross-sectional, observational study was conducted in a Tertiary Care Centre, R.G. Kar Medical College and Hospital, Kolkata, West Bengal, India after approval from Institutional Ethical Committee (IEC- ECR/322/Inst/WB/2013), for a total duration of six months from October 2019 to April 2020.
Inclusion criteria: The study consisted of patients who had undergone Modified Radical Mastectomy (MRM) in the institute and specimen sent for histopathological evaluation in Department of Pathology during the study period after obtaining the informed consent.
Exclusion criteria: Cases that had received Neo-Adjuvant Chemotherapy (NACT) with no proper tumour foci, no residual tumour or abundant necrosis were excluded from the study population.
A total number of 20 cases were found suitable for the present study within the stipulated time frame. Formalin-Fixed Paraffin-Embedded (FFPE) sections were examined by routine Haematoxylin and Eosin (H&E) stain; histologic type, grade by Elston Ellies Modification of Bloom Richardson Grading (BR Grade), staging and nodal status were evaluated and recorded [11]. Blocks suitable for immunohistochemical analysis of Estrogen Receptor (ER), Progesterone Receptor (PR), HER2/neu, Ki-67 and MCM2 were selected.
The IHC analysis was done using following antibodies: MCM2 (Rabbit Monoclonal IHC Antibody, EP40 (PathnSitu); Lot number R05053NAX), ER (Rabbit Monoclonal Antibody-Cell Marque- Lot number 1504203D), PR (Rabbit Monoclonal Antibody-Cell Marque-Lot number 1514106B), HER2/neu (Mouse Monoclonal Antibody-Cell Marque- Lot number 1515602B) and Ki-67 (Mouse Monoclonal IgG1 Antibody, GM001-PathnSitu). The chromogen used was 3,3′-Diaminobenzidine (DAB). Specificities of anti-MCM2 were confirmed using normal colonic mucosa based on the expression data in the human protein atlas database [Table/Fig-1] [12].
Positive control for MCM2 in colonic mucosa (100x magnification, IHC).
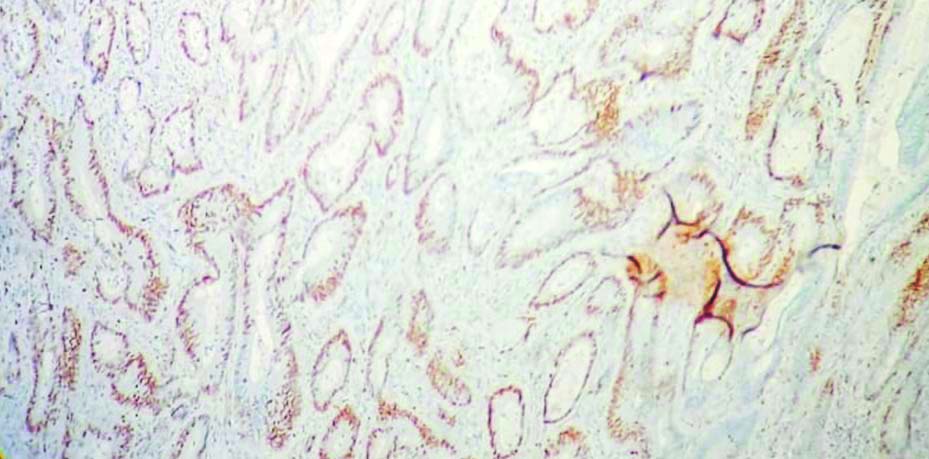
The stained IHC slides were examined under light microscope. The MCM2 expression was recorded by visual method as percentage of positive nuclear stains in hotspot areas (range: 0%-100%) [13]. Similar method was used to record Ki-67 expression status. For reporting ER, PR and HER2/neu status, the World Health Organisation (WHO) recommendations (2019) have been followed [11].
Statistical Analysis
All the data were tabulated in a Master Sheet and analysed using IBM Statistical Package for the Social Sciences Version 25.0 software. Descriptive statistics like mean, median, mode were used wherever applicable. Pearson’s Chi-square test (χ2) and Fisher’s Exact test were used to calculate statistical significance, where p-value is considered less than 0.05 as significant. Pearson’s Correlation Coefficient (R) and Relative Risk (RR) were also used wherever applicable.
Results
The study population belonged to the age group of 41-55 years amongst 10 cases (50%). The histologic type of Invasive Breast Carcinoma No Special Type (IBC-NST) included 18 out of 20 cases. Two other cases were that of metaplastic carcinoma. Majority of 12 cases (60%) were of BR grade 3 and 15 cases (75%) were of Locally Advanced Breast Carcinomas (LABC). The cases with positive nodal status were 12, whereas in four cases the nodal status was indeterminate (Nx). There were 5 ER positive (25%), 3 PR positive (15%) and 2 HER2 positive (10%) cases. Molecular classification showed that a majority of 14 cases (70%) were TNBC, three were Luminal B, two were Luminal A and one was HER2 positive (non luminal). [Table/Fig-2] summarises all the above observations.
Distribution of study population based on demographic variables and various immunohistologic parameters.
| Demographic variables | Frequency (n) | Percentage (%) |
|---|
| Age (years n=20) |
| ≤40 | 3 | 15 |
| 41-55 | 10 | 50 |
| 56-70 | 7 | 35 |
| Histologic type (n=20) |
| IBC-NST | 18 | 90 |
| Others (Metaplastic carcinoma) | 2 | 10 |
| BR Grade (n=20) |
| Grade 2 | 8 | 40 |
| Grade 3 | 12 | 60 |
| Stage (n=20) |
| Early | 5 | 25 |
| LABC | 15 | 75 |
| Nodal status (n=16) |
| Node positive | 12 | 75 |
| Node negative | 4 | 25 |
| Molecular types (n=20) |
| TNBC | 14 | 70 |
| Luminal A | 2 | 10 |
| Luminal B | 3 | 15 |
| HER2 positive (non luminal) | 1 | 5 |
IBC NST: Invasive breast carcinoma no special type; LABC: Locally advanced breast carcinomas; TNBC: Triple negative breast cancers; BR Grade: Bloom richardson grading
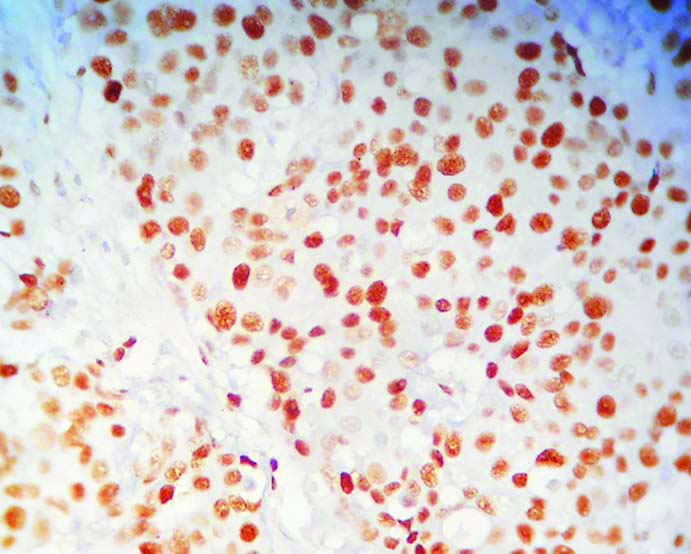
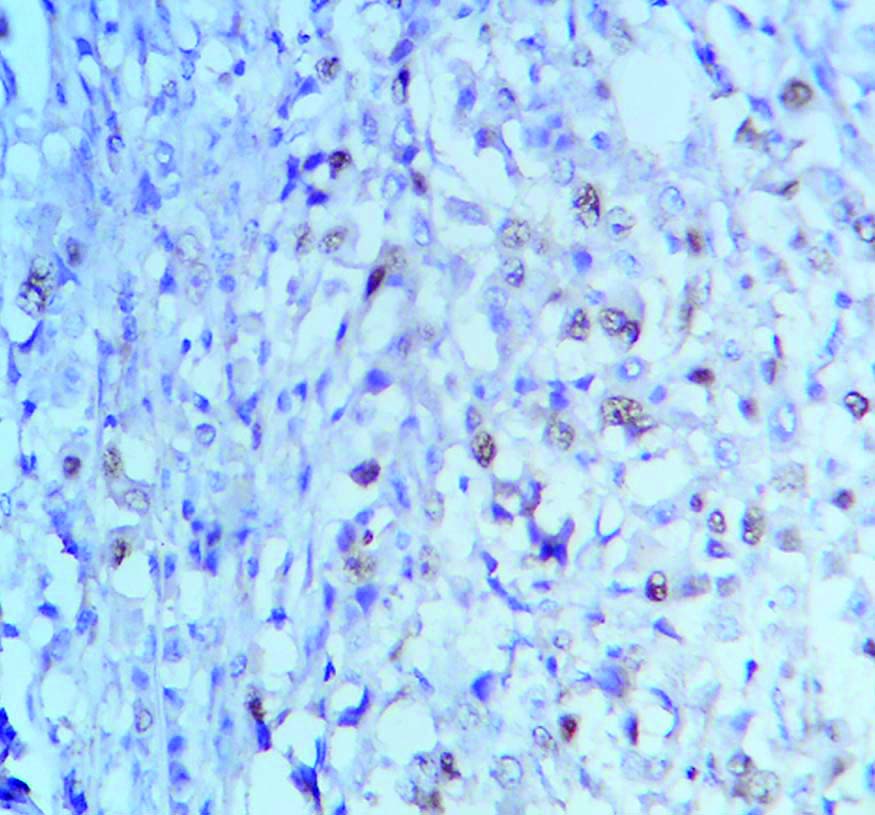
The mean MCM2 expression amongst the study population was 66.5% and median was 70%. For the purpose of grading, median value has been taken as cut-off [14]. So, an expression of <70% has been graded as low MCM2 expression and similarly ≥70% taken as high MCM2 expression [Table/Fig-3,4]. Following this criterion, 60% of the study population, i.e., 12 cases showed a high MCM2 expression. As per study conducted by Lombardi A et al., 20% is taken as cut-off for Ki-67 expression [5], which is also the median value in the present case. Cut-off >20% is graded as high Ki-67 expression and similarly, ≤20% is considered as low Ki-67 expression [Table/Fig-5]. Accordingly, 55% (11 out of 20 cases) showed low Ki-67 proliferation index in this study population, mean value of expression being 31.5%.
High MCM2 expression in BR Grade 3 breast cancer. (400x magnification);
Low MCM2 expression in BR Grade 2 breast cancer. (400x magnification).
High Ki-67 expression in TNBC. (400x magnification).
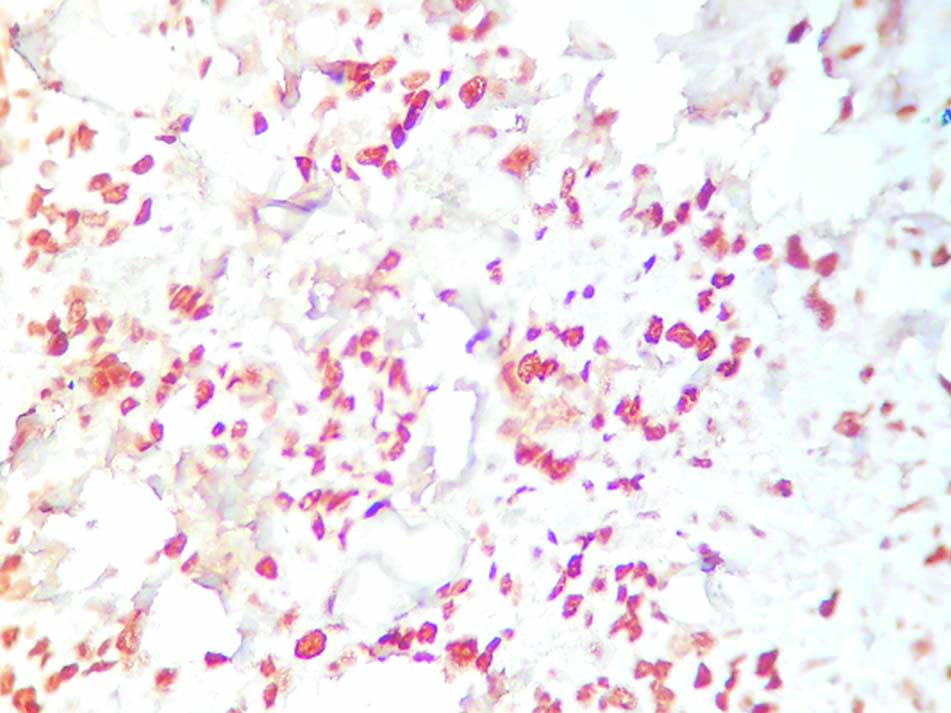
On performing Chi-square test, it was seen that high MCM2 expression was associated with high histologic grade of the tumour and the difference was statistically significant (p<0.05). Similar association was seen between high MCM2 expression and hormone receptors (ER/PR) negative tumours, with p-values less than 0.05 in both the cases. The TNBC cases also showed a very high MCM2 expression and the relationship was statistically significant. Such significant association (p<0.05) was also seen between high MCM2 and concurrently high Ki-67 expressions, with a weak but positive correlation (Pearson’s correlation coefficient (R)= 0.4318). The Ki-67 was also seen to be significantly high amongst high histologic grade and triple negative cases [Table/Fig-6].
Statistical associations between Ki-67 and MCM2 expressions and histologic type, grade, stage, nodal status and molecular variants of breast carcinoma.
| MCM2 | High MCM2 (≥70%) | Low MCM2 (<70%) | p-value (significant at <0.05) | Comment |
|---|
| Histologic type |
| IBC-NST | 10 | 8 | 0.4947 (Fisher-Exact Test) | Not significant |
| Others (Metaplastic carcinoma) | 2 | 0 |
| BR Grade |
| Grade 2 | 2 | 6 | 0.0090 | Significant |
| Grade 3 | 10 | 2 |
| Stage |
| Early | 2 | 3 | 0.2918 | 1. Not significant.2. r (Pearson’s correlation coefficient)=0.2645 (weak positive correlation).3. RR=1.33 |
| LABC | 10 | 5 |
| Lymph Node status |
| Positive | 7 | 5 | 0.5509 | Not significant |
| Negative | 3 | 1 |
| ER status |
| Positive | 0 | 5 | 0.0036 (Fisher’s-Exact Test) | Significant |
| Negative | 12 | 3 |
| PR status |
| Positive | 0 | 3 | 0.0491 (Fisher’s-Exact Test) | Significant |
| Negative | 12 | 5 |
| Molecular types |
| TNBC | 11 | 3 | 0.009 | Significant |
| Others | 1 | 5 |
| Ki-67 index |
| High (>20%) | 8 | 1 | 0.0171 | 1. Significant2. r=0.4318 (weak but positive correlation) |
| Low (≤20%) | 4 | 7 |
| Ki-67 | High Ki-67 (>20%) | Low Ki-67 (≤20%) | p-value (significant at <0.05) | Comment |
| BR Grade | | | | |
| Grade 2 | 1 | 7 | 0.0171 | Significant |
| Grade 3 | 8 | 4 |
| Stage |
| Early | 2 | 3 | 0.7952 | 1. Not significant2. R=0.5986 (moderate positive correlation)3. RR=1.07 |
| LABC | 7 | 8 |
| Lymph node status |
| Positive | 6 | 6 | 0.3827 | 1. Not significant2. RR=1.29 |
| Negative | 1 | 3 |
| Molecular types |
| TNBC | 9 | 5 | 0.0141 (Fisher-Exact Test) | Significant |
| Others (Luminal A, Luminal B, HER2 positive) | 0 | 6 |
Statistical tests used are- Chi-square test; Fisher’s-exact test; RR: Relative risk; r: Pearson’s correlation coefficient
No significant association was seen between MCM2 expression and histologic type, stage and nodal status of the cases. The Ki-67 expression and stage and nodal status of the patients were also not significantly associated. However, weak but positive correlation (r=0.2645) was seen between high MCM2 expression and advanced stage of the disease with a RR=1.33 >1. This indicates that a higher MCM2 expression is predisposed to have an advanced stage of cancer. Similar moderate positive correlation (r=0.5986) and RR=1.07 >1 was also seen between high Ki-67 expression and late stage of the disease. Risk ratio of 1.29 indicates that high Ki-67 index cases have higher risk of node positive diseases. All these statistical conclusions have been summarised in [Table/Fig-6].
Discussion
Breast cancer is one of the leading causes of cancer-related mortalities and deaths round the world, with very high incidence rate [15]. The majority of study population belonged to the age group of 41-55 years which corroborated with other study populations [16,17]. A major population in the present study was of locally advanced disease (Stage III), with no stage IV cases as the metastatic status of our cases were unknown. This was discordant with the findings of Kim EJ et al., where majority were stage I cases of breast cancer [18]. This can be attributed to the fact that there might be lack of awareness amongst women regarding breast cancer and screening by self-breast examination that leads to late presentation of the disease. Hospital bias may also be one of the contributing factors, where only the critically ill patients with advanced disease were referred from periphery to tertiary centre for further management. A substantial proportion of 70% was TNBC in this case, which was in concordance with Thakur KK et al., who evaluated the alarming burden of TNBCs among Indian population, influenced by various socio-demographic and genetic factors [19].
Statistical significance was established between high MCM2 expression and higher histologic grades of breast cancers. Similar association was also established by Bukholm IR et al., and many other studies [20-23]. Such statistical association was also seen between high MCM2 expression and the aggressive triple negative phenotype of breast carcinoma, which was also seen by Yousef EM et al., and others [1,7,21]. Tumours having high Ki-67 expression, which are aggressive in nature were also seen to have high MCM2 expressions as was also demonstrated by Wojnar A et al., and others [24,25]. High MCM2 was also associated with increased risk (RR>1) for advanced stage of disease, although statistical significance could not be established possibly due to small sample size of the study. However, previous works with a higher study population could conclusively prove that higher MCM2 expression was associated with node positive and advanced pTNM staging [26]. The Ki-67 was seen to be expressed more in higher histologic grades, as was established in various previous works [27-29]. Just like MCM2, TNBCs were also seen to have a high Ki-67 expression, which is concordant with Keam B et al., [30]. High Ki-67 cases were seen to have a higher risk of advanced stage and node positive disease as compared to low Ki-67 proliferative index, which is concordant with the findings of de Azambuja E et al., [31]. However, statistical significance could not be established between grades of Ki-67 expression and their adverse prognostic factors, small study population being the main contributory factor in this case.
Limitation(s)
Thus, it can be seen that small study population is the major limiting factor in this case. In many cases, statistical association could not be established due to small sample size. Also, the study needs to be conducted on a larger population for generalising the outcome and implementing it in our routine practice.
Conclusion(s)
Thus, it can be concluded by saying that MCM2 is a predictor for adverse prognostic factors like high histologic grade, high Ki-67 index and triple negative phenotypes. Its high expression also comes with increased risk of advanced stage of disease. The Ki-67 is also a predictor of poor outcome with increased risk of LABCs and node positive cases and its association with higher histologic grade and triple negative cases. However, in few scenarios statistical significance could not be established, small sample size being the main limiting factor.
The MCM2 can serve as an attractive alternative as a predictor of adverse outcomes, guiding a tailored strategy among patients and also avoid harmful overtreatments. Just like MCM2 has proved its worth as a novel therapeutic target in colon and breast cancers, its conclusive role in breast carcinogenesis may pave the way for newer therapeutic agents in treating more aggressive cancers like the triple negative subtypes, which are resistant to the conventional therapies.
IBC NST: Invasive breast carcinoma no special type; LABC: Locally advanced breast carcinomas; TNBC: Triple negative breast cancers; BR Grade: Bloom richardson grading
Statistical tests used are- Chi-square test; Fisher’s-exact test; RR: Relative risk; r: Pearson’s correlation coefficient