Introduction
The global prevalence of Diabetes Mellitus (DM) is estimated to increase to 10.4% (642 million) by 2040, that is, one in 10 adults will have diabetes [1]. Upto 90-95% of adults with diabetes have type 2 diabetes making it the most prevalent form of diabetes worldwide [1]. India, the largest country in the South-east Asia region with more than 65 million adults with diabetes, has the second highest number of cases in the world after China [2]. In India, the prevalence of diabetes is 10.4% in Tamil Nadu, 5.3% in Jharkhand, 13.6% in Chandigarh and 8.4% in Maharashtra [3]. The prevalence will have economic, social and healthcare impacts especially in developing countries, which have around 80% of people with diabetes [2]. This results in a significant burden due to increased morbidity and mortality, decreased life expectancy, and reduced quality of life, as well as individual and national income losses [4].
Apart from widely known complications like myocardial infarction, stroke, renal failure and visual impairment, patients with diabetes may develop lesser known musculoskeletal complications which may affect joints, soft tissues, nerves, muscles or tendons [5]. Some musculoskeletal conditions are uniquely seen in people with diabetes like limited joint mobility and diabetic muscular infarction, some are more prevalent in the diabetic population like Dupuytren’s contracture, Adhesive Capsulitis (AC), Neuropathic arthropathy, Flexor tenosynovitis and Diffuse Idiopathic Skeletal Hyperostosis (DISH). Some stem from other complications of diabetes, like peripheral neuropathy, while others seem to be directly caused by the metabolic abnormality. The association of osteoarthritis and Carpal Tunnel Syndrome (CTS) with diabetes has been proposed but not proven yet [5-7].
Kaka B et al., calculated the prevalence of musculoskeletal disorders in patients with diabetes as 58.15%, with the hand (33.05%) and shoulder (31.6%) being the most commonly affected areas [8]. Ramchurn N et al., found prevalence of musculoskeletal disorders to be 75% in diabetic patient’s vs 53% in controls [9]. Individuals with diabetes are more prone to developing musculoskeletal problems with long lasting symptoms and poor prognosis. They show higher pain levels, poorer functional outcomes, reduced quality of life and a reduced response to treatment compared to those without diabetes [10]. Due to differences in underlying mechanisms for patients with diabetes, it is not clear if the recommendations for treatment of musculoskeletal problems can be equally applied to patients with diabetes.
Physiotherapy plays a pivotal role in the management of diabetes and the various musculoskeletal disorders listed above. Exercises directed at the involved joint are helpful in alleviating pain and improving Range Of Motion (ROM), strength and function. Electrotherapeutical modalities like Transcutaneous Electrical Nerve Stimulation (TENS), Ultrasound, short wave diathermy, interferential therapy, are also used for pain relief. Inspite of being the first line of management for treating musculoskeletal disorders like AC, limited joint mobility, dupuytren’s contracture, trigger finger, CTS etc., and having proven efficacy in their management, the effectiveness of physiotherapy in diabetes related musculoskeletal disorders is still not clear. Despite a high prevalence of musculoskeletal disorders in diabetes, studies related to physiotherapy for musculoskeletal disorders in diabetes are very few. Thus, the present review was planned to study physiotherapy management in diabetes related musculoskeletal disorders.
Literature Search
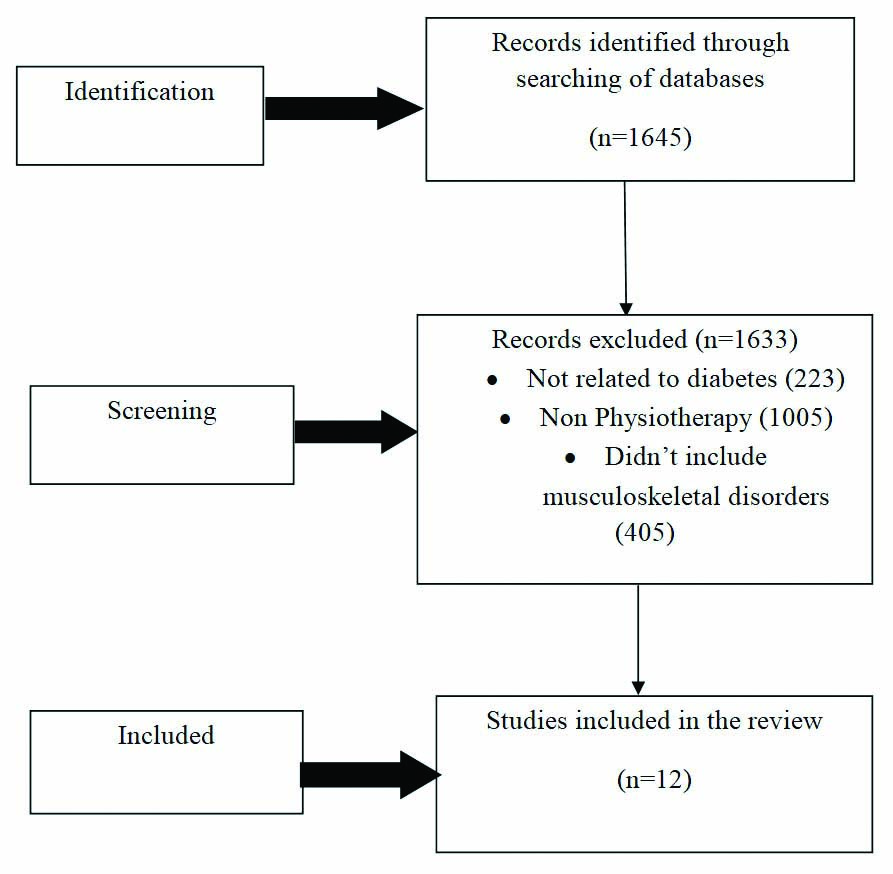
A comprehensive electronic search of databases like PubMed, Google Scholar, physiotherapy Evidence Database (PEDro), Cochrane Library was carried out. The terms ‘physiotherapy’ with ‘musculoskeletal disorders’ (problems/complications), ‘Diabetes’ (diabetes mellitus/type 2 DM), ‘AC’ (frozen shoulder, periarthritis), ‘Dupuytren’s contracture’ (disease), ‘Limited joint mobility’ (cheiroarthropathy), ‘CTS’, ‘median neuropathy’, ‘Flexor tenosynovitis’ (trigger finger), ‘Neuro osteoarthropathy’ (Charcot’s osteoarthropathy) ‘Reflex Sympathetic Dystrophy (RSD)’ (Sudeck’s dystrophy), ‘Diabetic muscle infarction’, ‘DISH’ were used for the search with Booleans ‘AND’/‘OR’/‘NOT’. The last date for searching was 31st December 2020. Filter for year of publication was applied from 1/1/2001 to 31/12/2020. Studies which were a part of the ‘references’ section of included studies were also hand searched. The inclusion criteria was: 1) Studies published in the last 20 years, i.e., from 1st January 2001 to 31st December 2020; 2) Studies published in English language; 3) Studies carried out on human participants; 4) Studies that implemented one or a combination of physiotherapy management techniques for musculoskeletal disorders in patients with diabetes including manual therapy, electrotherapy, exercise therapy etc.,; 5) Studies whose full-text was available. Studies were excluded if: 1) Management other than physiotherapy management like corticosteroid injections, Manipulation Under Anaesthesia (MUA) was used for treatment; 2) Physiotherapy management in postoperative conditions of musculoskeletal disorders were reported; 3) Only abstracts were available. [Table/Fig-1] outlines the methods for the review. [Table/Fig-2] shows the number of articles viewed against the keywords in PubMed.
Literature search results.
Number of articles viewed in PubMed.
| Sr. No. | Keywords | Number of articles viewed in pubmed |
|---|
| 1. | ‘Physiotherapy’ AND ‘Musculoskeletal disorders’ AND ‘Diabetes’ | 300 |
| 2. | ‘Physiotherapy’ AND ‘Musculoskeletal disorders’ AND ‘Diabetes mellitus’ | 175 |
| 3. | ‘Physiotherapy’ OR ‘Musculoskeletal disorders’ AND ‘Diabetes mellitus’ | 12,195 |
| 4. | ‘Physiotherapy’ AND ‘Diabetes mellitus’ OR ‘Musculoskeletal complications’ | 16,220 |
| 5. | ‘Physical therapy’ AND ‘Diabetes mellitus’ AND ‘Musculoskeletal complications’ | 49 |
| 6. | ‘Physical therapy’ AND ‘Diabetes mellitus’ OR ‘Musculoskeletal complications’ | 21,824 |
| 7. | ‘Physical therapy’ AND ‘Musculoskeletal disorders’ AND ‘Diabetes mellitus’ | 375 |
| 8. | ‘Physical therapy’ AND ‘Diabetes mellitus’ NOT ‘Musculoskeletal complications’ | 8529 |
| 9. | Physiotherapy’ AND ‘Diabetes mellitus’ NOT ‘Musculoskeletal complications’ | 2925 |
| 10. | ‘Physiotherapy’ AND ‘Musculoskeletal disorders’ NOT ‘Diabetes mellitus’ | 22,042 |
| 11. | ‘Physiotherapy’ AND ‘Adhesive capsulitis’ AND ‘Diabetes’ | 38 |
| 12. | ‘Physical therapy’ AND ‘Adhesive capsulitis’ AND ‘Diabetes mellitus’ | 24 |
| 13. | ‘Physiotherapy’ AND ‘Adhesive capsulitis’ NOT ‘Diabetes’ | 461 |
| 14. | ‘Physical therapy’ AND ‘Adhesive capsulitis’ NOT ‘Diabetes’ | 627 |
| 15. | ‘Physical therapy’ AND ‘Frozen shoulder’ NOT ‘Diabetes’ | 646 |
| 16. | ‘Physical therapy’ AND ‘Frozen shoulder’ AND ‘Diabetes mellitus’ | 24 |
| 17. | ‘Physical therapy’ AND ‘Periarthritis’ AND ‘Type 2 diabetes’ | 0 |
| 18. | ‘Physiotherapy’ AND ‘Periarthritis’ AND ‘Diabetes’ | 3 |
| 19. | ‘Physiotherapy’ AND ‘Carpal tunnel syndrome’ AND ‘Diabetes’ | 10 |
| 20. | ‘Physiotherapy’ AND ‘Carpal tunnel syndrome’ NOT ‘Diabetes’ | 349 |
| 21. | ‘Physical therapy’ AND ‘Carpal tunnel syndrome’ AND ‘Diabetes’ | 15 |
| 22. | ‘Physical therapy’ AND ‘Median neuropathy’ AND ‘Diabetes’ | 25 |
| 23. | ‘Physiotherapy’ AND ‘Limited joint mobility’ AND ‘Diabetes’ | 27 |
| 24. | ‘Physiotherapy’ AND ‘Cheiroarthropathy’ AND ‘Diabetes mellitus’ | 0 |
| 25. | ‘Physiotherapy’ AND ‘Dupuytren’s contracture’ AND ‘Diabetes’ | 6 |
| 26. | ‘Physiotherapy’ AND ‘Dupuytren’s contracture’ NOT ‘Diabetes’ | 55 |
| 27. | ‘Physiotherapy’ AND ‘Dupuytren’s contracture’ | 61 |
| 28. | ‘Physical therapy’ AND ‘Dupuytren’s contracture’ | 68 |
| 29. | ‘Physiotherapy’ AND ‘Flexor tenosynovitis’ AND ‘Diabetes’ | 2 |
| 30. | ‘Physiotherapy’ AND ‘Trigger finger’ AND ‘Diabetes’ | 1 |
| 31. | ‘Physiotherapy’ AND ‘Trigger finger’ | 43 |
| 32. | ‘Physiotherapy’ AND ‘Trigger finger’ NOT ‘diabetes’ | 42 |
| 33. | ‘Physical therapy’ AND ‘Trigger finger’ NOT ‘diabetes’ | 50 |
| 34. | ‘Physiotherapy’ AND ‘Diffuse idiopathic skeletal hyperostosis’ AND ‘Diabetes’ | 2 |
| 35. | ‘Physiotherapy’ AND ‘Diffuse idiopathic skeletal hyperostosis’ | 10 |
| 36. | ‘Physical Therapy’ AND ‘Diffuse idiopathic skeletal hyperostosis’ | 18 |
| 37. | ‘Physical therapy’ AND ‘Diffuse idiopathic skeletal hyperostosis’ NOT ‘diabetes’ | 15 |
The search yielded 1645 papers. Twelve primary research papers were included after studies not related to diabetes, studies not including physiotherapy treatment and musculoskeletal disorders were removed. Except for AC, studies related to physiotherapy in other conditions related to diabetes were few to none.
Adhesive Capsulitis
The AC is characterised by a painful and progressive limitation of active and passive ROM, resulting in functional disability. It is also known as Frozen shoulder [11,12]. It is more common in people with diabetes with a prevalence ranging from 10-76% in type 1 and 7-30% in type 2 diabetes [11,13-15]. Patients with diabetes are five times more likely to have AC [14]. In the different states of India the prevalence of AC in diabetic patients ranges from: 13.1% in Jammu & Kashmir (J&K) to 45.31% in Kolkata [16-18]. Around the world the prevalence of AC is: 25% in the UK, 10.9% in Pakistan and 12.5% in Morocco [9,15,19]. In Ethiopia, the cumulative prevalence of shoulder and hand complications was 16.6% and in type 2 DM was 21.3% [20]. AC is associated with age and duration. Diabetic patients have a tendency for more severe symptoms and are resistant to treatment [13]. Pathologically, in patients with diabetes, there is glycosylation of collagen fibers of the capsule and impaired circulation of the small capillaries [11].
Non surgical treatment options in AC include physiotherapy, oral or intra-articular corticosteroids, Non Steroidal Anti-Inflammatory Drugs (NSAIDs), acupuncture, hydrodilatation. Arthroscopic capsular release and MUA are reserved for those who do not improve with non surgical treatments [11-14,21-25]. Early treatment interventions are supported by moderate to strong evidences vs. low evidence for no treatment [11]. Physiotherapy being the choice of treatment for AC helps in reducing pain and improving shoulder ROM. Shortwave diathermy, ultrasound, or electrical stimulation combined with mobility and stretching exercises have been recommended (weak evidence). Joint mobilisation techniques of the glenohumeral joint (weak evidence) and stretching exercises (moderate evidence), have also been recommended [26]. Maund E et al., concluded in their review that short wave diathermy and high grade mobilisation may be more effective than home exercise and low grade mobilisation respectively [27]. [Table/Fig-3] shows the studies retrieved for physiotherapy in AC in patients with diabetes [11,28-36].
Physiotherapy management in Adhesive Capsulitis (AC) in patients with diabetes [11,28-36].
| Author and Year | Type of study | Objectives/Intervention | Outcomes assessed | Conclusions related to physiotherapy management |
|---|
| Sana’a AA et al., [11], 2019 | Systematic review | To see the effectiveness of one or combination of steroid injections, physiotherapy interventions, hydrodilatation, suprascapular nerve block and manipulation under anaesthesia in managing adhesive capsulitis in people with diabetes. | Any one of the following:Visual Analog Scale (VAS), Range Of Motion (ROM), Shoulder Pain and DisabilityIndex (SPADI), Constant Shoulder Score (CSS), American Shoulder and Elbow Surgeons (ASES), and shoulder test questionnaires. | Joint mobilisation and exercises, continuous passive motion with electrotherapy and low-level laser therapy showed better outcomes in pain, ROM, and function than other physiotherapeutic interventions. |
| Kyhlbäck M et al., [28], 2019 | Pre-post treatment design | Type 1 diabetic patients (n=10) received light-load exercises, muscle relaxation and shoulder co-ordination. The outcome was compared with the results from another observational study. | Short Form-36 questionnaire (SF-36), Shoulder movement impairment scale, Numerical Rating Scale (NRS), Shoulder Rating Questionnaire-Swedish version (SRQ-S). | The Physical Component Summary (PCS) of SF-36 showed significantly higher scores, persisting at six months follow up. Significant improvements were found in pain, shoulder function, and shoulder ROM. |
| Muthukrishnan R et al., [29], 2019 | Randomised controlled trial | Experimental group (n=10): Extracorporeal Shock Wave Therapy (ESWT) upto 2000 times, frequency 3 Hz, intensity of energy: according to participant’s tolerance of pain, one session per week.Control group (n=10): Ultrasound therapy (6-8 minutes), Hot packs (10-12 mins) thrice a week for four weeks. At twelfth week all participants received a follow-up session. Both groups received mobilisation and therapeutic exercises. | VAS, Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, Global Rating of Change (GROC) score. | Significant improvement was seen in EWST group after four weeks in pain with four sessions per month whereas control group achieved similar results in 12 sessions per month. |
| Mueller MJ et al., [30], 2018 | Prospective, randomised, controlled clinical trial | Shoulder Mobility program: Participants (n=25) given intervention of 30-60 minutes duration for three months. Home Exercise Program (HEP) consisted of end-range active shoulder mobility exercises, passive stretching of shoulder flexion and rotations and resisted shoulder motions. Minimum 03 stretches, 10 repetitions, 2 sets were performed everyday. Duration of home exercise was 10 to 15 minutes, 2 times per day.Wellness program: Participants (n=24) were seen four times over three months and instructed in blood sugar control, physical activity (150 min/wk of moderate intensity aerobic activity), foot care, and blood pressure control. | ROM of active shoulder flexion and SPADI. | Compared to wellness group participants in mobility group improved in active shoulder motion and reported reduced pain and disability which persisted nine months later. |
| Santoboni F et al., [31], 2017 | Observationalpilot trial | Fifty patients with diabetes received ESWT once a week for 3 weeks, 2,400 shots in an anterior-to-posterior direction on the anterior shoulder joint using a low/ moderate energy flux density (0.06–0.14 mJ/mm2) | VAS, CSS, Quick DASH. | ESWT was beneficial both acutely and chronically as significant improvements were seen in all outcomes at two months, and further improvement was seen at four and six months. |
| Ekim AA et. al, [32], 2016 | Prospective, randomised, clinical trial | Continuous Passive Motion (CPM) group (n = 20) and Conventional Physiotherapy (CPT) group (n = 21) received treatment for four weeks (five days per week). CPM was given for 1 h/day. CPT consisted of active stretching, ROM and pendular exercises 1h/day. 20 minutes hot-pack, 5 minutes therapeutic ultrasound (1.5 watt/cm2) and 20 minutes Transcutaneous Electrical Nerve Stimulation (TENS) was given to all patients HEP of pendulum and passive ROM exercises given for eight weeks after intervention. | VAS, Active and passive shoulder ROM, Constant Shoulder Score (CSS), SPADI. | At 4th and 12th week, VAS (at rest, in motion, and at night) active and passive abduction and flexion ROM, SPADI and Constant score showed statistically significant differences in both groups. CPM group showed more improvement than CPT group. |
| Youssef RA et al., [33], 2015 | Comparative study | Mulligan group (n=15): Passive accessory glide in the desired direction as the patient moved the arm actively. Three sets of 10 repetitions in each direction.Maitland group (n=15): Posterior, anterior and caudal oscillatory end-range glide of grade III or IV was given.Both the groups were treated three times a week, for six weeks. In addition, pendulum exercises in all directions for five minutes were also given. | Active shoulder flexion, abduction, internal and external rotation ranges, SPADI-Arabic version. | Both Mulligan and Maitland mobilisation were effective in reducing pain and dysfunction and improving mobility in the shoulder. But Mulligan mobilisation was more effective. |
| Soliman AS et al., [34], 2014 | Observational study | Group I (n=20) received Low Level Laser Therapy (LLLT). Group II (n=20) received reflexology in the form of thumb walk over the shoulder area on the bottom of the foot under the little toe. Both received treatment for 15 minutes.Therapy was given 3 times/week for eight weeks with 15 minutes of exercise. Stretching and pendular exercises were also given. All exercises were repeated 10 times. | Shoulder joint ROM. | There was significant improvement in ROM and reduction in pain with both LLLT and reflexology in diabetic patients with AC; LLLT was more effective. |
| Kyhlbäck M et al., [35], 2014 | Pre-post treatment design | Type 1 diabetic patients (n=10) received relaxation exercises of neck shoulder muscles, light-load muscle exercises, shoulder co-ordination, behavioural strategies to maintain a good posture. Outcome compared with the results from another observational study. | NRS, Shoulder Rating Questionnaire –Swedish version (SRQ-S), E-SES (Exercise-Self-Efficacy Scale). | Shoulder pain decreased and daily activities of patients improved with diabetes. |
| Düzgün I et.al, [36], 2012 | Observational study | group 1: Participants with primary AC with type 2 DM (n=12), group 2 Participants having only primary AC (n=38).Both groups received cold application, scapular mobilisation glenohumeral joint, posterior capsular stretching and theraband exercises progressively over four weeks and were evaluated at eight weeks. | Shoulder ROM,Constant’s score, VAS, shoulder muscle strength. | Pain decreased and ROM, functional activity and muscular strength improved in both groups.There were no differences in outcome measures between both the groups. |
Limited Joint Mobility Syndrome (LJMS)
It is a painless, non inflammatory limitation of the mobility of hands, feet, and large joints. Diabetic cheiroarthropathy or Stiff hand syndrome is a type of LJMS that affects the upper extremity. It generally starts as cutaneous changes around the metacarpophalangeal and Proximal Interphalangeal (PIP) joints of the fifth finger, and progresses to involve all fingers. Skin changes progress to thick, rigid and waxy appearance [7,37-42]. ROM limitation is characterised by an inability to fully flex or extend the fingers and sclerosis of tendon sheaths are also seen. Patients can be asymptomatic or can have pain, which increases with the use of the extremity [5,7,38,40-43]. “Prayer sign” and “table top test” may be positive in patients with LJMS [5,7,38,40-43].
Diabetic cheiroarthropathy prevalence ranges from 38-58% in type 1 DM and 45-76% in type 2 DM patients which increases with duration of diabetes [5,7,38,40-43]. In different studies in India, the prevalence of LJMS in diabetic patients ranges from 8.06% in Kerala to 37.5% in Kolkata [16-18]. Around the world, the prevalence ranges from 2.9% in Morocco to 63.1% in Jordan [6,9,15,19,44].
Biochemically there is an increase in glycosylation of collagen fibers; collagen crosslinking and increased formation of Advanced Glycosylation End products (AGEs), also there is altered collagen synthesis, decreased collagen degradation and diabetic microangiopathy [5,7,37,38,40,41,43].
According to Abate M et al., and Gerrits EG et al., there is no known curative treatment, other than optimising glycaemic control, as no drugs directly target LJMS. Only symptomatic therapy, like analgesics, NSAIDs or local corticosteroid injections, is given for pain relief. Surgery is indicated for severe contractures. To prevent or delay the development of progressive joint stiffness, stretching exercises may help [45,46]. Professional foot care and rocker bottom shoes are necessary to prevent development of diabetic foot ulcers in limited joint mobility of the lower limb [42,46].
Francia P et al., in their review described the relationship between LJMS and development of diabetic foot ulcers. They showed that physical therapy in the short term considerably improved the ankle and foot mobility in patients with LJMS and neuropathy but long term follow-up studies are required. Joint mobility, muscle performance and walking speed improved significantly with 12 weeks of exercise therapy in patients with LJMS which was comparable to controls [42].
Dupuytren’s contracture: Dupuytren’s contracture is a condition in which there is thickening and fibrosis of the palmar fascia, palmar and digital nodule formation and contractures develop in the digits. In DM predominantly the middle and ring fingers are involved, as opposed to involvement of ring and little fingers in non diabetics [5,7,38,40,41,43,47,48]. It has been reported in 16-42% of diabetic patients who often experience a milder form of disease [5,7,38-41,43,47,48]. Age and duration of diabetes are associated with an increased incidence of dupuytren’s contracture [38,41,47]. Local hypoxia results in release of free radicals which in turn affects fibroblasts that lead to production of altered collagen fibers [7].
The prevalence of dupuytren’s contracture in diabetic patients in India ranges from 2.26% in Kerala to 23% in J&K [16-18]. Around the world, the prevalence was between 0.5% in Morocco to 18.6% in Jordan [6,9,15,19,44].
Treatment consists of optimising glycaemic control and physiotherapy, which may be beneficial for early or mild cases. Intralesional steroid injection, radiotherapy and recently, injections of collagenase Clostridium histolyticum have been used for treatment [7,40-42,48]. Surgical intervention may be needed for severe cases.
Studies evaluating the effectiveness of physical therapy in dupuytren’s contracture are less and those in diabetes are fewer. A systematic review which mostly included case series, included ultrasound, splinting, frictional massage and heat treatment with joint stretching as part of early physical therapy treatment. Ultrasound given between 4 and 10 minutes weekly over five to eight weeks showed improvement in digital extension, hand span and grip strength. Splints worn at night also led to an increase in digital extension. There was an improvement of 200 in PIP joint contractures of two patients treated by a combination of extension splinting, stretching exercises and friction massage [49]. Cross frictional massage combined with maximal extension stretch of the wrist, hand and 4th and 5th digits given thrice weekly for eight weeks increased passive and active extension of digits, reduced visibility of palmar adhesions, and led to subjective improvements in hand function compared to stretching alone. In all the above studies as sample sizes were small, evidence to support the use of physical therapies was very limited and inconclusive [49,50].
A case series of four patients with diabetes having dupuytren’s contracture evaluated the effectiveness of Extra Corporeal Shock Wave Therapy (ECSWT) of 2000 impulses, 3 Hz, 1.24 mJ/mm2 applied for five sessions with seven days between and found that it reduced pain and tenderness [51].
Patients with PIP joint flexion contracture of 40° or more, following standard collagenase injection and cord rupture were given orthotic intervention and reverse blocking exercises for PIP joint extension and distal interphalangeal joint flexion. The contracture decreased 88% from the 1st week till four weeks [52].
Carpal Tunnel Syndrome (CTS)
Carpal Tunnel Syndrome seen in up to 20% of diabetic patients and is more common in women [5,38,39,41,43,44,47,48]. Its prevalence in diabetic patients increases with duration. Patients complain of burning, paresthesias, or sensory loss in the distribution of median nerve which in first three fingers and radial half of the fourth finger. In addition, pain which is aggravated by wrist flexion or extension and may awake patients from sleep, may also be present [5,38-41,43,47,48]. Tinel’s sign and Phalen’s test assist in clinical diagnosis. There may also be decreased thenar muscle strength and atrophy [5,38,39,41,43,44,47,48].
The prevalence of CTS in diabetic patients in India is: 11.1% in J&K, 20.3% in Kolkata, 0.32% in Kerala [16-18]. Around the world, it ranges from over 5% in Greece to 29.2% in Ethiopia [6,9,15,19,44].
Two theories regarding the mechanism of CTS in diabetes prevail. Firstly, connective tissue glycosylation increases collagen cross-linking, leading to stiffness and thickening of the transverse carpal ligament [47,48]. Secondly, polyneuropathy, due to microvascular changes of diabetes, increases the susceptibility of the median nerve to a compressive injury [5,38,39,41,43,44,47,48].
The CTS can be confirmed by Electromyogram/Nerve Conduction Velocity (EMG/NCV) tests. Management of CTS does not differ in diabetic and non diabetic patients. Conservative treatment involves wrist splints, (especially at night) NSAIDs, analgesics and corticosteroid injections initially. Release surgery with a 4-14-times greater frequency in diabetic patients might be required with often a worse postoperative recovery. This could be due to microangiopathy related impairment of peripheral nerve regeneration, dysfunction of macrophages and Schwann cells, and reduced expression of neurotrophic factors and their receptors in diabetes [5,38,39,41,43,44,47,48].
In a study on diabetic patients with CTS, manual therapy group improved more compared to modality group as shown in [Table/Fig-4] [53,54].
Physiotherapy management in Carpal Tunnel Syndrome (CTS) and diabetic muscle infarction in patients with diabetes [53,54].
| Author and Year | Type of study | Objectives/Intervention | Outcomes assessed | Conclusions related to physiotherapy management |
|---|
| Talebi GA et al., [53], 2018 | Randomised clinical trial | Group I, modality group (n=15) received Transcutaneous Electrical Nerve Stimulation (TENS) (frequency-80 Hz, pulse duration-60 μs, time -20 minutes) till a comfortable tingling sensation and therapeutic ultrasound (frequency-1 MHz, intensity-1 W/cm2, time-5 minutes) on the palmar surface of carpal tunnel.Group II, manual therapy (n=15) received manual therapy like mobilisation of carpal bone, transverse carpal ligament release, palmar fascia release of the hand, soft tissue manipulation in distal arm and proximal forearm and neural mobilisation (Shacklocks approach) 25 minutes, collectively, for each session.Both groups were treated three times a week for four weeks. | Primary: VAS Boston questionnaire consisting of Symptom Severity Scale (SSS) and Functional Status Scale (FSS), Secondary: Median neurodynamic test. | Manual therapy group improved significantly more in VAS, SSS, FSS and MNT whereas modality group changed only in VAS and SSS at the end of four weeks. |
| Onyenemezu I and Capitle E Jr [54], 2014 | Review | To know the treatment outcome and favorability of surgery, physiotherapy, or bed rest in diabetic muscle infarction. | Mean time to recovery, mean time to recurrence and one-year recurrence rate. | Mean time to recurrence for DMI was highest for bed rest (297 days) followed by physiotherapy (107 days) and surgery (30 days). The recurrence rate was least for bed rest (24%) followed by physiotherapy (44%) showing that physiotherapy had a treatment outcome comparable with that of bed rest. Judicious exercise regime undertaken at an appropriate time, can prevent deconditioning and promote muscle tissue regeneration. Optimal therapy for DMI may be a period of bed rest during the acute phase followed by gentle physiotherapy as tolerated. |
In a Cochrane review on non surgical treatment for CTS (2003) [54,55], Hand brace, (four weeks), Ultrasound treatment (seven weeks), Yoga (eight weeks) vs splinting, Carpal bone mobilisation (three weeks) vs no treatment showed significant improvement. Ergonomic keyboards versus control demonstrated equivocal results for pain and function. Magnet therapy, laser acupuncture, exercise or chiropractic care did not demonstrate symptom benefit when compared to placebo or control [54,55].
Flexor Tenosynovitis (Trigger Finger)
Flexor tenosynovitis (trigger finger), a frequent complication in patients with diabetes has a prevalence of 20% in diabetic population vs 2% in the general population. Individuals complains of catching sensation, stiffness, pain, or locking in the digit, with tenderness and palpable nodule usually over the metacarpophalangeal joint, and thickening along the affected flexor tendon sheath. Active or passive finger flexion may lead to locking. In diabetic patients trigger finger is seen bilaterally, involving multiple digits with relative sparing of index and small fingers and females are more affected. Age and duration of diabetes are significant contributing factors [5,7,38-41,43,47,48].
The prevalence of trigger finger in diabetic patients in India ranges from 2.58% in Kerala to 18.8% in Jammu & Kashmir [16-18]. Globally, the prevalence ranges from 3.8% in Pakistan to 29% in UK [6,9,15,19,44].
Physiotherapy, an accepted treatment for trigger fingers, consists of various modalities like heat, stretching and joint motion [55,56]. Apart from this, modification of activities, use of NSAIDs, splinting, corticosteroid injection into the tendon sheath are also used. In more severe cases, surgery is done [7,38]. Diabetic patients respond less well to corticosteroid injection and more often require surgery [7,38-41,47,48].
Salim N et al., compared the effectiveness of physiotherapy (wax therapy, ultrasound, stretching and massage) and corticosteroid injection in mild trigger fingers. At three months steroid injection group had 97.4% success rate vs. 68.6% in the physiotherapy group. At six months corticosteroid group had a significant recurrence rate of pain but not triggering whereas the physiotherapy group had neither recurrence of pain nor triggering [56]. A single session of one minute of dry needling was effective in decreasing pain and improving pinch power in patients with trigger finger [57]. An metacarpophalangeal joint blocking splint showed positive outcomes in 77% of subjects compared to 50% in the distal interphalangeal joint splint [58].
Diffuse Idiopathic Skeletal Hyperostosis (DISH)
The DISH, also known as, also known as Forestier’s disease and ankylosing hyperostosis, is characterised by diffuse calcification and ossification of the ligaments and enthuses. Tendon and ligament attachments get ossified at both spinal and extraspinal (skull, pelvis, greater trochanters, patellae, calcaneus, elbow) locations as well as hyperostosis may develop at bony prominences. The prevalence of DISH in type 2 DM ranges from 13-40%, vs. 2.2-3.5% in general population and is also commonly seen in obese people. Nearly one-half of patients with DISH have diabetes [5,7,38,40,41,43,44,48].
Patients are rarely symptomatic and DISH is diagnosed radiologically using Resnick and Niwayama criteria [7,48]. It most commonly affects the thoracic spine. It can cause spinal rigidity and impingement of nearby structures and nerves, resulting in hoarseness, stridor, sleep apnea, and dysphagia. The exact mechanism of DISH is not known. However, insulin, growth hormone, and insulin growth factor (IGF-1) are proposed as factors that promote bone growth in DISH [5,43]. For symptomatic patients, physiotherapy and analgesics are standard treatment options. Surgery is rarely performed, only in cases of pressure syndromes [5,38,40,41,43,48].
Al-Herz A et al., studied the effect of strengthening, mobility and stretching exercise on pain, ROM and function in persons with DISH. One hour of supervised exercises by a physiotherapist for 14 visits were given over eight weeks. The physical measures improved but were not statistically significant [59].
Neuropathic Osteoarthropathy (Charcot Osteoarthropathy)
Neuropathic osteoarthropathy, also known as Charcot osteoarthropathy, is a progressive destructive process affecting the bones and joints. It is commonly seen in DM, with ankle, metatarsophalangeal and tarsometatarsal joints being affected. It is characterised by acute and chronic phases. Initially, the foot is warm, edematous, and erythematous. Pain may or may not be present, depending on the degree of neuropathy. Later on abnormal pressure on the plantar surface may lead to rocker bottom deformity. Calluses may form which are liable to ulcerations especially in the mid-foot which may ultimately lead to amputation [5,7,38,40,41,43,48].
The neurotraumatic theory states that repeated mechanical trauma to a joint that is insensitive to pain results in microfractures. According to the neurovascular theory changes result from a neurally initiated vascular reflex leading to hyperaemia and increased bone resorption by osteoclasts [37]. A recent hypothesis, is an excessive inflammatory response to traumas, mediated by pro-inflammatory cytokines.
Management consists of optimising glycaemic control and regular foot care and review. For those already affected, weight-bearing limitations for at least three months are put in place for healing. Also, appropriate orthosis and crutches can relieve pressure on the affected joints during ambulation. NSAIDs, calcitonin, and bisphosphonates may be used. Surgical treatment may be required when conservative treatment fails [5,7,38,41,43,48].
Diabetic Muscle Infarction
Diabetic muscle infarction (aseptic myonecrosis, infarcted myonecrosis), a rare complication of DM is mostly associated with longstanding poorly controlled DM, particularly in patients with type 1 DM. Patients, without any prior history of trauma, present with acute pain with swelling in a muscle with appearance of palpable mass that persists at rest and worsens with exercise. The thigh muscles are most commonly affected [41]. Diagnosis is mainly on the basis of clinical presentation and radiological finding, where Magnetic Resonance Imaging (MRI) is the test of choice.
Muscle infarction is most likely caused by arteriosclerosis and diabetic microangiopathy. Endothelial dysfunction and hypercoagulability due to alteration of coagulation-fibrinolytic system in DM have been proposed as a potential pathogenic mechanism. The treatment involves bed rest, analgesics, and aggressive control of DM. Vigorous physical therapy may lead to an exacerbation, hence should be avoided. Patients usually recover with bed rest [5,37,38,41,43,48,54].
Reflex Sympathetic Dystrophy (Sudeck’s Atrophy/Shoulder Hand Syndrome/Complex Regional Pain Syndrome)
Reflex Sympathetic Dystrophy is characterised by diffuse or localised pain, either in the upper or lower extremity, occuring spontaneously or after minimal trauma. Swelling, vasomotor disturbances, and trophic skin changes are usually associated with it. DM may predispose one to RSD. Treatments, including analgesics, physiotherapy, intravenous bisphosphonates, calcitonin, oral corticosteroids, and sympathetic ganglion blocks have been used with variable results [5,38,43,48].
Physiotherapy clearly plays a critical role in functional restoration. Physical therapy is the cornerstone and first line treatment for Complex Regional Pain Syndrome (CRPS) [60]. Physiotherapy can help patients increase their ROM, flexibility, and strength through gentle progressive exercises. A more recent approach to exercise is directed towards activation of cortical networks which are presumed to be altered in chronic pain and CRPS. Graded Motor Imagery (GMI), a sequential set of brain exercises, comprises laterality training, imagined hand movements, and mirror feedback therapy that leads to pain reduction and increase in functional capacity of CRPS patients.
In children with CRPS, physiotherapy combined with cognitive-behavioural therapy demonstrated significant improvement in pain and function in majority of subjects. Aquatic therapy can also be quite valuable to CRPS patients [61]. Physiotherapy for upper-limb CRPS-I is likely to have a beneficial impact on the disorder and on how patients cope with the condition (level 2) [62].
Conclusion(s)
Physiotherapy, often a first line of treatment, plays a vital role in the management of DM related musculoskeletal disorders. Maintaining an optimal glycaemic control is equally important as these conditions deteriorate with increasing glucose levels. Studies on physiotherapy management in diabetes related musculoskeletal disorders were very few to none in some conditions. Good quality studies which specifically study the effects of physiotherapy interventions in diabetes related musculoskeletal disorders are needed so that early treatment can be initiated and quality of life can be improved.
[1]. Cavan D, Fernandes J, Makaroff L, Ogurtsova K, Webber S, International Diabetes FederationIDF Diabetes Atlas 2015 7th edBrussels, BelgiumInternational Diabetes Federation [Google Scholar]
[2]. Ramachandran A, Snehalatha C, Ma RC, Diabetes in south-east Asia: An updateDiabetes Research and Clinical Practice 2014 103(2):231-37.10.1016/j.diabres.2013.11.01124300015 [Google Scholar] [CrossRef] [PubMed]
[3]. Mohan V, Kaur T, Anjana, RM, Pradeepa R, ICMR-INdia DIABetes [INDIAB] Study-Phase 1: Final Report (2008-2011)Accessed from https://main.icmr.nic.in/sites/default/files/reports/ICMR_INDIAB_PHASE_I_FINAL_REPORT.pdf [Google Scholar]
[4]. Mohan V, Seedat YK, Pradeepa R, The rising burden of diabetes and hypertension in southeast Asian and African regions: Need for effective strategies for prevention and control in primary health care settingsInternational Journal of Hypertension 2013 2013:40908310.1155/2013/40908323573413 [Google Scholar] [CrossRef] [PubMed]
[5]. Merashli M, Chowdhury TA, Jawad AS, Musculoskeletal manifestations of diabetes mellitusQJM: An International Journal of Medicine 2015 108(11):853-57.10.1093/qjmed/hcv10626025688 [Google Scholar] [CrossRef] [PubMed]
[6]. Douloumpakas I, Pyrpasopoulou A, Triantafyllou A, Sampanis CH, Aslanidis S, Prevalence of musculoskeletal disorders in patients with type 2 diabetes mellitus: A pilot studyHippokratia 2007 11(4):216 [Google Scholar]
[7]. Silva MB, Skare TL, Musculoskeletal disorders in diabetes mellitusRev Bras Reumatol 2012 52(4):594-609.10.1590/S0482-5004201200040001022885425 [Google Scholar] [CrossRef] [PubMed]
[8]. Kaka B, Maharaj SS, Fatoye F, Prevalence of musculoskeletal disorders in patients with diabetes mellitus: A systematic review and meta-analysisJournal of Back and Musculoskeletal Rehabilitation 2019 32(2):223-35.10.3233/BMR-17108630248032 [Google Scholar] [CrossRef] [PubMed]
[9]. Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, Upper limb musculoskeletal abnormalities and poor metabolic control in diabetesEuropean Journal of Internal Medicine 2009 20(7):718-21.10.1016/j.ejim.2009.08.00119818294 [Google Scholar] [CrossRef] [PubMed]
[10]. Kalra S, Kalra B, Sharma N, Sharma S, Physiotherapy in the management of diabetes mellitus: Utility and benefitsThe Internet Journal of Pain, Symptom Control and Palliative Care 2010 8(1):03-04.10.5580/1e44 [Google Scholar] [CrossRef]
[11]. Sana’a AA, Nazari G, Bobos P, MacDermid JC, Overend TJ, Faber K, Effectiveness of nonsurgical interventions for managing adhesive capsulitis in patients with diabetes: A systematic reviewArchives of Physical Medicine and Rehabilitation 2019 100(2):350-65.10.1016/j.apmr.2018.08.18130268804 [Google Scholar] [CrossRef] [PubMed]
[12]. Hsu JE, Anakwenze OA, Warrender WJ, Abboud JA, Current review of adhesive capsulitisJournal of Shoulder and Elbow Surgery 2011 20(3):502-14.10.1016/j.jse.2010.08.02321167743 [Google Scholar] [CrossRef] [PubMed]
[13]. Whelton C, Peach CA, Review of diabetic frozen shoulderEuropean Journal of Orthopaedic Surgery & Traumatology 2018 28(3):363-71.10.1007/s00590-017-2068-829094212 [Google Scholar] [CrossRef] [PubMed]
[14]. Ramirez J, Adhesive capsulitis: Diagnosis and managementAmerican Family Physician 2019 99(5):297-300. [Google Scholar]
[15]. Majjad A, Errahali Y, Toufik H, Djossou JH, Ghassem MA, Kasouati J, Musculoskeletal disorders in patients with diabetes mellitus: A cross-sectional studyInternational Journal of Rheumatology 2018 2018:383987210.1155/2018/383987230018643 [Google Scholar] [CrossRef] [PubMed]
[16]. Bhat TA, Dhar SA, Dar TA, Naikoo MA, Naqqash MA, Bhat A, The musculoskeletal manifestations of type 2 diabetes mellitus in a Kashmiri populationInternational Journal of Health Sciences 2016 10(1):57-68.10.12816/003122227004058 [Google Scholar] [CrossRef] [PubMed]
[17]. Kumar T, Das A, Rheumatological manifestations in diabetes mellitus: Distribution and associated factorsIOSR Journal of Dental and Medical Sciences 2016 15(6):51-54.10.9790/0853-1508112932 [Google Scholar] [CrossRef]
[18]. Mathew AJ, Nair JB, Pillai SS, Rheumatic-musculoskeletal manifestations in type 2 diabetes mellitus patients in south IndiaInternational Journal of Rheumatic Diseases 2011 14(1):55-60.10.1111/j.1756-185X.2010.01587.x21303482 [Google Scholar] [CrossRef] [PubMed]
[19]. Kidwai SS, Wahid L, Siddiqi SA, Ghauri I, Sheikh I, Upper limb musculoskeletal abnormalities in type 2 diabetic patients in low socioeconomic strata in PakistanBMC Research Notes 2013 6(1):1610.1186/1756-0500-6-1623327429 [Google Scholar] [CrossRef] [PubMed]
[20]. Fasika S, Abebe SM, Kebede AG, The prevalence of shoulder and hand complications and associated factors among diabetic patients at University of Gondar Teaching Referral Hospital in Northwest EthiopiaJournal of Diabetes Research & Clinical Metabolism 2013 4(12):13-14.10.7243/2050-0866-2-8 [Google Scholar] [CrossRef]
[21]. Ewald A, Adhesive capsulitis: A reviewAmerican Family Physician 2011 83(4):417-22. [Google Scholar]
[22]. Lamplot JD, Lillegraven O, Brophy RH, Outcomes from conservative treatment of shoulder idiopathic adhesive capsulitis and factors associated with developing contralateral diseaseOrthopaedic Journal of Sports Medicine 2018 6(7):232596711878516910.1177/232596711878516930023406 [Google Scholar] [CrossRef] [PubMed]
[23]. Blanchard V, Barr S, Cerisola FL, The effectiveness of corticosteroid injections compared with physiotherapeutic interventions for adhesive capsulitis: A systematic reviewPhysiotherapy 2010 96(2):95-107.10.1016/j.physio.2009.09.00320420956 [Google Scholar] [CrossRef] [PubMed]
[24]. Levine WN, Kashyap CP, Bak SF, Ahmad CS, Blaine TA, Bigliani LU, Nonoperative management of idiopathic adhesive capsulitisJournal of Shoulder and Elbow Surgery 2007 16(5):569-73.10.1016/j.jse.2006.12.00717531513 [Google Scholar] [CrossRef] [PubMed]
[25]. Woods DA, Loganathan K, Recurrence of frozen shoulder after Manipulation Under Anaesthetia (MUA) the results of repeating the MUAThe Bone & Joint Journal 2017 99(6):812-17.10.1302/0301-620X.99B6.BJJ-2016-1133.R128566402 [Google Scholar] [CrossRef] [PubMed]
[26]. Kelley MJ, Shaffer MA, Kuhn JE, Michener LA, Seitz AL, Uhl TL, Shoulder pain and mobility deficits: Adhesive capsulitis: Clinical practice guidelines linked to the international classification of functioning, disability, and health from the Orthopaedic Section of the American Physical Therapy AssociationJournal of Orthopaedic & Sports Physical Therapy 2013 43(5):A01-31.10.2519/jospt.2013.030223636125 [Google Scholar] [CrossRef] [PubMed]
[27]. Maund E, Craig D, Suekarran S, Neilson A, Wright K, Brealey S, Management of frozen shoulder: A systematic review and cost-effectiveness analysisHealth Technol Assess 2012 16(11):01-264.10.3310/hta1611022405512 [Google Scholar] [CrossRef] [PubMed]
[28]. Kyhlbäck M, Söderlund A, Thierfelder T, Elmgren Frykberg G, Physiotherapy treatment of the diabetic shoulder: Health-related quality of life and measures of shoulder function regarding patients with type 1 diabetesDisability and Rehabilitation 2019 41(12):1435-42.10.1080/09638288.2018.143017729363341 [Google Scholar] [CrossRef] [PubMed]
[29]. Muthukrishnan R, Rashid AA, Al-Alkharji F, The effectiveness of extracorporeal shockwave therapy for frozen shoulder in patients with diabetes: Randomised control trialJournal of Physical Therapy Science 2019 31(7):493-97.10.1589/jpts.31.49331417208 [Google Scholar] [CrossRef] [PubMed]
[30]. Mueller MJ, Sorensen CJ, McGill JB, Clark BR, Lang CE, Chen L, Effect of a shoulder movement intervention on joint mobility, pain, and disability in people with diabetes: A randomised controlled trialPhysical Therapy 2018 98(9):745-53.10.1093/ptj/pzy07029893977 [Google Scholar] [CrossRef] [PubMed]
[31]. Santoboni F, Balducci S, D’Errico V, Haxhi J, Vetrano M, Piccinini G, Extracorporeal shockwave therapy improves functional outcomes of adhesive capsulitis of the shoulder in patients with diabetesDiabetes Care 2017 40(2):e12-13.10.2337/dc16-206327899492 [Google Scholar] [CrossRef] [PubMed]
[32]. Ekim AA, İnal EE, Gönüllü E, Hamarat H, Yorulmaz G, Mumcu G, Continuous passive motion in adhesive capsulitis patients with diabetes mellitus: A randomised controlled trialJournal of Back and Musculoskeletal Rehabilitation 2016 29(4):779-86.10.3233/BMR-16068927002662 [Google Scholar] [CrossRef] [PubMed]
[33]. Youssef RA, Ibrahim AM, Ayad EK, Mulligan mobilisation is more effective in treating diabetic frozen shoulder than the Maitland techniqueInt J Physiother 2015 2(5):804-10.10.15621/ijphy/2015/v2i5/78238 [Google Scholar] [CrossRef]
[34]. Soliman AS, Mahmoud AM, Serry ZM, Dawood FG, Therapeutic effects of low-level laser and reflexology on adhesive capsulitis in elderly type 2 diabetic patientsAsian J Pharm Clin Res 2014 7(5):317-21. [Google Scholar]
[35]. Kyhlbäck M, Schröder Winter H, Thierfelder T, Söderlund A, Physiotherapy treatment of the diabetic shoulder: A longitudinal study following patients with diabetes and shoulder pain using a pre-post treatment designDisability and Rehabilitation 2014 36(7):556-62.10.3109/09638288.2013.80459323781906 [Google Scholar] [CrossRef] [PubMed]
[36]. Düzgün ı, Baltacı G, Atay ÖA, Manual therapy is an effective treatment for frozen shoulder in diabetics: An observational studyJoint Diseases and Related Surgery 2012 23(2):094-99. [Google Scholar]
[37]. Arkkila PE, Gautier JF, Musculoskeletal disorders in diabetes mellitus: An updateBest Practice & Research Clinical Rheumatology 2003 17(6):945-70.10.1016/j.berh.2003.11.00115123045 [Google Scholar] [CrossRef] [PubMed]
[38]. Kim RP, Edelman SV, Kim DD, Musculoskeletal complications of diabetes mellitusClinical Diabetes 2001 19(3):132-35.10.2337/diaclin.19.3.132 [Google Scholar] [CrossRef]
[39]. Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM, Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitusThe American Journal of Medicine 2002 112(6):487-90.10.1016/S0002-9343(02)01045-8 [Google Scholar] [CrossRef]
[40]. Wyatt LH, Ferrance RJ, The musculoskeletal effects of diabetes mellitusThe Journal of the Canadian Chiropractic Association 2006 50(1):43-50. [Google Scholar]
[41]. Sözen T, Başaran NÇ, Tınazlı M, Özışık L, Musculoskeletal problems in diabetes mellitusEuropean Journal of Rheumatology 2018 5(4):258-65.10.5152/eurjrheum.2018.1804430388074 [Google Scholar] [CrossRef] [PubMed]
[42]. Francia P, Anichini R, Seghieri G, De Bellis A, Gulisano M, History, prevalence and assessment of limited joint mobility, from stiff hand syndrome to diabetic foot ulcer prevention: A narrative review of the literatureCurrent Diabetes Reviews 2018 14(5):411-26.10.2174/157339981366617081614273128814244 [Google Scholar] [CrossRef] [PubMed]
[43]. Smith L, Burnet S, McNeil J, Musculoskeletal manifestations of diabetes mellitusBritish Journal of Sports Medicine 2003 37(1):30-35.10.1136/bjsm.37.1.3012547740 [Google Scholar] [CrossRef] [PubMed]
[44]. Mustafa KN, Khader YS, Bsoul AK, Ajlouni K, Musculoskeletal disorders of the hand in type 2 diabetes mellitus: Prevalence and its associated factorsInternational Journal of Rheumatic Diseases 2016 19(7):730-35.10.1111/1756-185X.1261726259148 [Google Scholar] [CrossRef] [PubMed]
[45]. Abate M, Schiavone C, Salini V, Andia I, Management of limited joint mobility in diabetic patientsDiabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2013 6:197-207.10.2147/DMSO.S3394323690694 [Google Scholar] [CrossRef] [PubMed]
[46]. Gerrits EG, Landman GW, Nijenhuis-Rosien L, Bilo HJ, Limited joint mobility syndrome in diabetes mellitus: A minireviewWorld Journal of Diabetes 2015 6(9):1108-12.10.4239/wjd.v6.i9.110826265997 [Google Scholar] [CrossRef] [PubMed]
[47]. Fitzgibbons PG, Weiss AP, Hand manifestations of diabetes mellitusThe Journal of Hand Surgery 2008 33(5):771-75.10.1016/j.jhsa.2008.01.03818590861 [Google Scholar] [CrossRef] [PubMed]
[48]. Al-Homood IA, Rheumatic conditions in patients with diabetes mellitusClinical Rheumatology 2013 32(5):527-33.10.1007/s10067-012-2144-823247555 [Google Scholar] [CrossRef] [PubMed]
[49]. Ball C, Izadi D, Verjee LS, Chan J, Nanchahal J, Systematic review of non-surgical treatments for early dupuytren’s diseaseBMC Musculoskeletal Disorders 2016 17(1):345Doi: 10.1186/s12891-016-1200-y10.1186/s12891-016-1200-y27526686 [Google Scholar] [CrossRef] [PubMed]
[50]. Christie WS, Puhl AA, Lucaciu OC, Cross-frictional therapy and stretching for the treatment of palmar adhesions due to Dupuytren’s contracture: A prospective case studyManual Therapy 2012 17(5):479-82.10.1016/j.math.2011.11.00122123331 [Google Scholar] [CrossRef] [PubMed]
[51]. Abdulsalam AJ, Shehab D, Abd AE, Abraham M, High-energy focused extracorporeal shockwave therapy relieved pain in Dupuytren’s disease: A series of seven handsEuropean Journal of Physical and Rehabilitation Medicine 2019 55(6):862-64.Doi: 10.23736/S1973-9087.18.05498-910.23736/S1973-9087.18.05498-930370754 [Google Scholar] [CrossRef] [PubMed]
[52]. Skirven TM, Bachoura A, Jacoby SM, Culp RW, Osterman AL, The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints caused by Dupuytren disease and treated with collagenase injectionThe Journal of Hand Surgery 2013 38(4):684-89.10.1016/j.jhsa.2013.01.03823474162 [Google Scholar] [CrossRef] [PubMed]
[53]. Talebi GA, Saadat P, Javadian Y, Taghipour M, Manual therapy in the treatment of carpal tunnel syndrome in diabetic patients: A randomised clinical trialCaspian Journal of Internal Medicine 2018 9(3):283-89.Doi: 10.22088/cjim.9.3.283 [Google Scholar]
[54]. Onyenemezu I, Capitle Jr E, Retrospective analysis of treatment modalities in diabetic muscle infarctionOpen Access Rheumatology: Research and Reviews 2014 6:01-06.10.2147/OARRR.S5375727790029 [Google Scholar] [CrossRef] [PubMed]
[55]. O’Connor D, Marshall SC, Massy-Westropp N, Pitt V, Non-surgical treatment (other than steroid injection) for carpal tunnel syndromeCochrane Database of Systematic Reviews 2003 1:CD003219Doi: 10.1002/14651858.CD00321910.1002/14651858.CD00321912535461 [Google Scholar] [CrossRef] [PubMed]
[56]. Salim N, Abdullah S, Sapuan J, Haflah NH, Outcome of corticosteroid injection versus physiotherapy in the treatment of mild trigger fingersJournal of Hand Surgery (European Volume) 2012 37(1):27-34.10.1177/175319341141534321816888 [Google Scholar] [CrossRef] [PubMed]
[57]. Azizian M, Bagheri H, Olyaei G, Shadmehr A, Okhovatpour MA, Dehghan P, Effects of dry needling on tendon-pulley architecture, pain and hand function in patients with trigger finger: A randomised controlled trial studyJournal of Physical Therapy Science 2019 31(4):295-98.10.1589/jpts.31.29531036998 [Google Scholar] [CrossRef] [PubMed]
[58]. Tarbhai K, Hannah S, von Schroeder HP, Trigger finger treatment: A comparison of 2 splint designsThe Journal of Hand Surgery 2012 37(2):243-49.10.1016/j.jhsa.2011.10.03822189188 [Google Scholar] [CrossRef] [PubMed]
[59]. Al-Herz A, Snip JP, Clark B, Esdaile JM, Exercise therapy for patients with diffuse idiopathic skeletal hyperostosisClinical Rheumatology 2008 27(2):207-10.10.1007/s10067-007-0693-z17885726 [Google Scholar] [CrossRef] [PubMed]
[60]. Bussa M, Guttilla D, Lucia M, Mascaro A, Rinaldi S, Complex regional pain syndrome type I: a comprehensive reviewActa Anaesthesiologica Scandinavica 2015 59(6):685-97.10.1111/aas.1248925903457 [Google Scholar] [CrossRef] [PubMed]
[61]. Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, Complex regional pain syndrome: Practical diagnostic and treatment guidelinesPain Medicine 2013 14(2):180-229.10.1111/pme.1203323331950 [Google Scholar] [CrossRef] [PubMed]
[62]. Perez RS, Zollinger PE, Dijkstra PU, Thomassen-Hilgersom IL, Zuurmond WW, Rosenbrand KC, Evidence based guidelines for complex regional pain syndrome type 1BMC Neurology 2010 10(1):01-04.10.1186/1471-2377-10-2020356382 [Google Scholar] [CrossRef] [PubMed]