Lesions of urinary bladder constitute one of the most common urologic condition and accounts for significant morbidity and mortality [1,2]. It is the second most common neoplasm of genitourinary tract next to prostate carcinoma [3]. As these bladder tumours represent a heterogeneous group with different subtypes and behavioural patterns, these neoplasms pose biologic, clinical, diagnostic and therapeutic challenges to both urologist and uropathologist [4,5]. According to data of Indian cancer registry, it is the ninth most common cancer [6]. Among men, it is the fourth most common cancer and eighth most common malignancy in women [7].
The major reported risk factors for Transitional Cell Carcinoma (TCC) includes increased exposures to tobacco smoke, occupational toxins, and environmental sources of heavy metals such as arsenic [8,9]. The primary sign associated with bladder carcinoma is usually haematuria [10], which is often non specific and requires further investigation. For an accurate diagnosis of urinary bladder lesions simultaneous data from urology, radiology and surgical pathology labs are very essential. Cystoscopy remains as the primary screening tool for patients which allows a direct visualisation of the bladder mucosa and facilitates biopsies of the suspected lesions [11], but to provide an accurate and definitive diagnosis histopathology is the only resort.
The present study aimed to identify the frequency of bladder lesions, with emphasis on histomorphological features of bladder lesions.
Materials and Methods
This was a descriptive retrospective study conducted over a period of six months starting from July 2020 to December 2020 and data were collected from records for the period of January 2015 to December 2019 carried out in the Department of Pathology, Government Medical College, Trivandrum, Kerala, India, after obtaining Institutional Ethical Clearance, (HEC .No.04/86/2020/MCT dated 26.06.2020). A total of 742 cases were included in the study.
Inclusion and exclusion criteria: Record of all the patients who visited the Urology Outpatient Department (OPD) with lower urinary tract symptoms and obstructive urinary symptoms were included. Bladder biopsies were taken from the cystoscopically abnormal areas and tumours. Autolysed specimens and biopsies with no submucosa and at least part of muscularis propria were excluded from the study.
Study Procedure
The cystoscopic bladder biopsies taken were fixed in 10% buffered formalin and then processed with embedding in paraffin wax. Multiple sections of 3-5 μ thick were taken and stained with regular Haematoxylin and Eosin stain (H&E). The histological features were studied and relevant findings were noted. Patient’s history, clinical diagnoses were also obtained from the patient’s record file and histopathological requisition forms. Standard diagnostic criteria’s were used to diagnose based in morphological findings. The nomenclature of the lesions was as per World Health Organization (WHO) classification of Tumours of the Urinary System and Male Genital Organs. Fourth Edition [12] and American Joint Committee on Cancer-Tumour-Node-Metastasis (TNM) staging manual [13].
Statistical Analysis
Data was entered in excel sheet and was analysed. Categorical variables were summarised as percentage.
Results
A total of 742 cystoscopic bladder biopsy cases were studied, age range of the cases in the study is from 18-86 years with most common age group of 61-80 years and sex ratio of 6.9:1. Haematuria, was the most common clinical manifestation in 739 cases (99.59%) followed by increased frequency and dysuria. Personal habits like smoking, alcoholism and tobacco intake were seen in 549 (73.98%), 163 (21.96%) and 30 (4.04%) patients respectively in the present study. Fever, nocturia, limb oedema were relatively less common clinical manifestation [Table/Fig-1].
Clinical features of cases in present study.
| Clinical features | Number of cases | Percentage (%) |
|---|
| Haematuria | 739 | 99.59 |
| Increased frequency | 423 | 57 |
| Urgency | 234 | 31.53 |
| Dysuria | 312 | 42.04 |
| Nocturia | 30 | 4.04 |
| Incomplete voiding | 317 | 42.72 |
| Abdominal pain | 331 | 44.60 |
| Fever | 26 | 3.50 |
| Lower limb oedema | 12 | 1.61 |
| Weight loss | 124 | 16.71 |
Out of the 742 cases, studied 46 cases were non neoplastic lesions and 688 were neoplastic lesions and eight cases were of metastatic malignancies [Table/Fig-2]. Most common non neoplastic lesion was non specific cystitis (17 cases), followed by 13 cases of cystitis glandularis of which six cases showed associated intestinal metaplasia [Table/Fig-3].
Non neoplastic lesions were most commonly males and in age group of 41-60 years constituting 15 cases (47.82%).
Among neoplastic lesions, urothelial carcinoma was the predominant type 678 cases (98.54%) and was most commonly seen in age group of 61-80 years constituting 431 cases (62.64%). These neoplastic lesions were more common among males, 608 cases (81.94 %) with M:F ratio of 7.6:1 [Table/Fig-4].
Histopathological spectrum of non neoplastic and neoplastic urinary bladder lesions in present study.
| Sl. No. | Histomorphological diagnosis | No. of cases (n) | Percentage (%) |
|---|
| I | Non neoplastic | 46 | 6.19 |
| a | Non specific cystitis | 17 | 36.95 |
| b | Follicular cystitis | 4 | 8.69 |
| c | Eosinophilic cystitis | 2 | 4.34 |
| d | Polypoid cystitis | 3 | 6.52 |
| e | Cystitis glandularis | 13 | 28.26 |
| f | Cystitis cystica | 4 | 8.69 |
| g | Diverticulitis | 2 | 4.34 |
| h | Endometriosis | 1 | 2.17 |
| II | Neoplastic | 688 | 92.72 |
| A | Urothelial Neoplasms | 678 | 98.54 |
| a | Urothelial carcinoma in situ | 3 | 0.43 |
| b | PUNLMP | 21 | 3.05 |
| c | Urothelial papilloma | 4 | 0.58 |
| d | Inverted Urothelial papilloma | 1 | 0.14 |
| e | Nephrogenic adenoma | 2 | 0.29 |
| f | Non invasive papillary urothelial neoplasm low grade | 332 | 48.25 |
| g | Non invasive papillary urothelial neoplasm high grade | 166 | 24.12 |
| h | Invasive Urothelial Carcinoma (IUC) | 149 | 21.65 |
| 1) Papillary | | |
| * no differentiation | 127 | 85.23% |
| * Papillary with squamous differentiation | 21 | 14.58 |
| * Papillary with glandular differentiation | 1 | 0.69 |
| 2) IUC microcystic variant | 2 | 1.34 |
| 3) IUC clear cell variant | 2 | 1.34 |
| 4) IUC sarcomatoid variant | 1 | 0.69 |
| B | Squamous cell carcinoma | 1 | 0.13 |
| C | Urachal carcinoma | 2 | 0.26 |
| D | Neuroendocrine tumours | 2 | 0.26 |
| a | Small cell carcinoma | 1 | 50 |
| b | Paraganglioma | 1 | 50 |
| 5 | Mesenchymal tumours | 5 | 0.67 |
| a | Leiomyosarcoma | 2 | 40 |
| b | Inflammatory myofibroblastic tumour | 2 | 40 |
| c | Leiomyoma | 1 | 20 |
| III | Metastatic tumours | 8 | 1.07 |
| Total | 742 | |
PUNLMP: Papillary urothelial neoplasm of low malignant potential
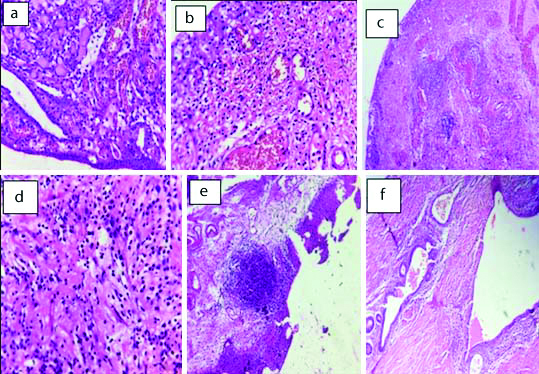
Non neoplastic lesions : a) Cystitis glandularis (H&E 40X); b) Cystitis cystica (H&E 40X); c) Polypoid Cystitis (H&E 40X); d) Eosinophilic cystitis (H&E 40X); e) Follicular cystitis (H&E, 40X); f) Endometriosis (H&E 40X)
Age wise distribution of urothelial Non neoplastic lesions in the present study.
| Age group (years) | Non neoplastic lesions (n=46) | Percentage (%) | Neoplastic lesions (n=688) | Percentage (%) |
|---|
| Males | Females | Males | Females |
|---|
| <20 | 1 | 0 | 2.17 | 3 | 0 | 0.43 |
| 20-40 | 6 | 3 | 19.56 | 12 | 8 | 2.90 |
| 41-60 | 15 | 7 | 47.82 | 169 | 25 | 28.19 |
| 61-80 | 13 | 0 | 28.26 | 387 | 44 | 62.64 |
| >80 | 1 | 0 | 2.17 | 37 | 3 | 5.66 |
Non invasive papillary urothelial neoplasm low grade was the pre dominant type, 332 cases (48.25%) followed by non invasive papillary urothelial neoplasm high grade 166 cases (24.12%) and invasive urothelial carcinomas 149 cases (21.65%) [Table/Fig-5]. All invasive urothelial carcinomas demonstrated definite deep muscle invasion, which was totally absent in both low and high grade non invasive papillary urothelial neoplasm [Table/Fig-6].
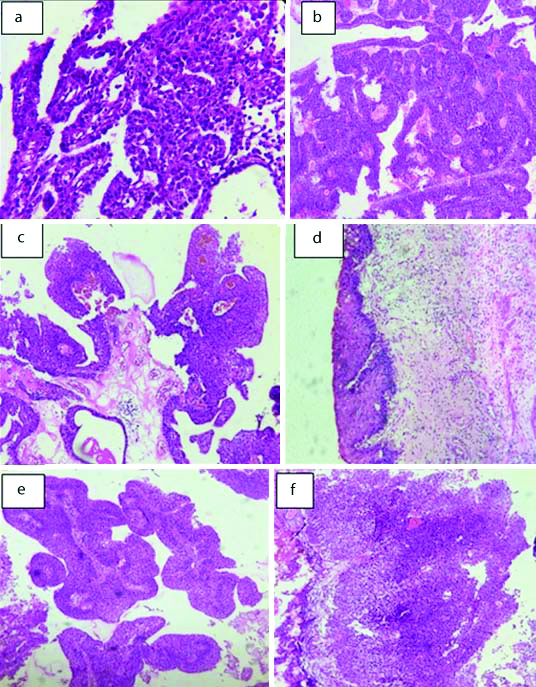
Non invasive neoplastic lesions: a) Nephrogenic adenoma (H&E, 20X); b) Urothelial papilloma (H&E, 20X); c) Papillary Urothelial Neoplasm of Low Malignant Potential (H&E, 20X); d) Carcinomain situ (H&E, 20X); e) Low grade papillary urothelial carcinomaf); f) High grade papillary urothelial carcinoma (H&E, 20X).
Age wise distribution of urothelial neoplasms in the present study.
| Age group (years) | Urothelial neoplasms |
|---|
| Non invasive urothelial neoplasms (n=529) | Invasive urothelial neoplasms (n=149) |
|---|
| Urothelial carcinoma in situ | PUNLMP | Urothelial papilloma | Inverted urothelial papilloma | Nephrogenic adenoma | LGPUC (n=332) | HGPUC (n=166) | Papillary/conventional | Microcystic | Clear cell | Sarcomatoid variant |
|---|
| <20 | - | 5 | - | - | - | 3 | - | - | - | - | - |
| 20-40 | - | 12 | - | - | 2 | 12 | 1 | - | - | - | - |
| 41-60 | 1 | 4 | 2 | 1 | - | 104 | 46 | 31 | - | - | 1 |
| 61-80 | 2 | 0 | 2 | - | - | 192 | 107 | 109 | 2 | 2 | - |
| >80 | - | 0 | | - | - | 21 | 12 | 4 | - | - | - |
HGPUC: High grade papillary urothelial carcinoma; LGPUC: Low grade papillary urothelial carcinoma; PUNLMP: Papillary urothelial neoplasm of low malignant potential
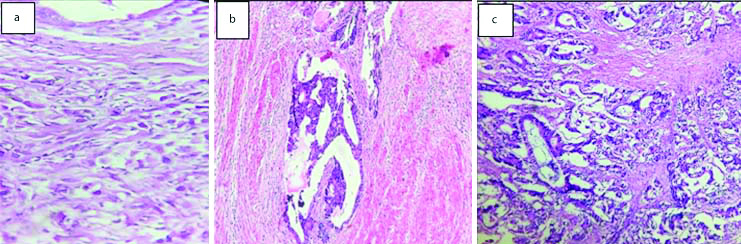
A total of 149 invasive urothelial carcinomas studied, of which 127 cases (85.23%) were invasive papillary urothelial carcinomas. Of these 21 cases of invasive papillary urothelial carcinomas showed squamous differentiation (14.58%) and one case with glandular differentiation (0.69%) [Table/Fig-7]. Two cases each of invasive urothelial carcinomas (1.34%) were microcystic and clear cell variants and 1 case of (0.69%) sarcomatoid variant was studied [Table/Fig-8].
Invasive urothelial neoplasms: a) Papillary type (H&E 40X); b) Squamous differentiation (H&E 40X); c) Glandular differentiation (H&E 40X); d) Tumour cells with perineural invasion (H&E 40X).
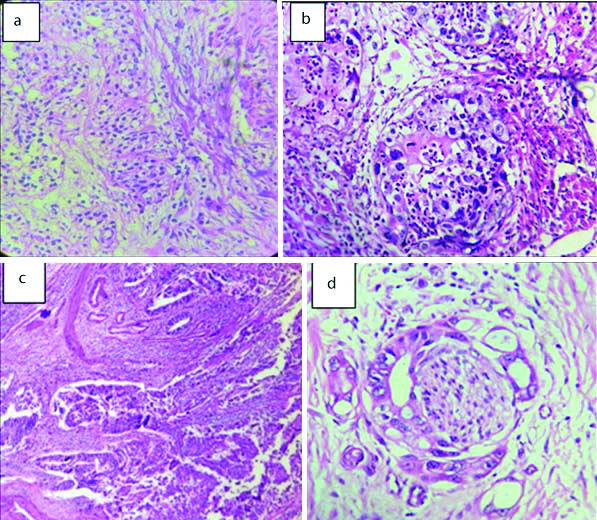
Variants of Invasive urothelial neoplasms. Sarcomatoid carcinoma bladder showing: (a) muscle invasion. (H&E 20X); (b) Pleomorphic spindly cells in Sarcomatoid carcinoma bladder (H&E, 40X).
Out of 149 cases of invasive urothelial neoplasms in the present study, 3 (2.01%) were showing invasion into lamina propria (superficially invasive bladder carcinoma) and 42 (28.18 %) cases showed invasion into superficial muscle layer and 102 cases (68.45%) shows invasion into deep muscle layer and 2 cases (1.34%) showed serosal penetration.
Authors encountered with two cases of urachal carcinoma with clinical and radiological findings favouring the same, one was seen in a 69-year-old male primary urachal adenocarcinoma, who presented with haematuria and lower abdominal lump. Radical cystoprostatectomy with en-bloc resection of urachus and umbilectomy was done. Grossly, an ulceroproliferative growth was seen involving both urachus and dome of urinary bladder and other case of urachal carcinoma was associated with mucinous areas in a 23-year-old female. The tumour was very fragile and had mucin pools in it. Histologically, it was mucinous adenocarcinoma with presence of intracellular and extracellular mucin (mucicarmine positive).
One case of pure squamous cell carcinoma was seen in a 48-year-old female, authors confirmed the diagnosis after excluding any primary from the female genital system.
Two cases of neuroendocrine neoplasms were seen in males over 60 years of age (a case of small cell carcinoma and one paraganglioma) which was confirmed with immunohistochemical markers of neuroendocrine differentiation [Table/Fig-9].
Neuroendocrine neoplasms: Small cell carcinoma bladder showing: a) Muscle invasion (H&E, 20X); b) Serosalinvasion (H&E, 20X); c) Paraganglioma (H&E, 20X).
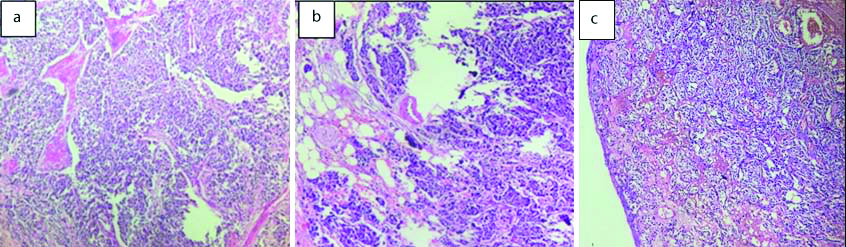
Among the five cases of mesenchymal neoplasms two cases were leiomyosarcoma, two cases of inflammatory myofibroblastic tumour and one case of leiomyoma. Leiomyosarcoma was seen more in elderly age group than inflammatory myofibroblastic tumour and leiomyoma. Except for one case of leiomyosarcoma, rest all mesenchymal neoplasms were seen in females [Table/Fig-10].
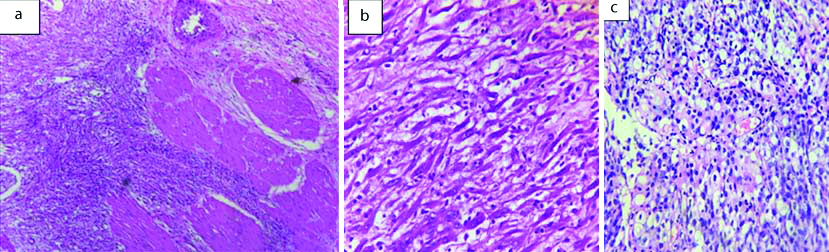
Mesenchymal neoplasms of bladder: a) Leiomyoma (H&E, 20x); b) Inflammatory myofibroblastic tumour (H&E, 40x); c) Leiomyosarcoma (H&E, 20x).
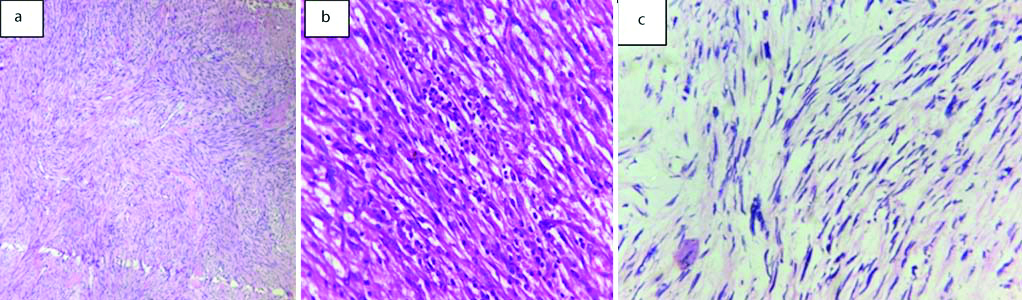
Eight cases were metastatic malignancy to urinary bladder with gastrointestinal tract being the most common primary site (75%), one case was a prostatic metastasis to bladder and other was from invasive lobular carcinoma breast [Table/Fig-11].
Metastatic lesions of bladder: a) Invasive lobular carcinoma metastasis (H&E, 20X); b) metastasis from colonic carcinoma (H&E, 20X); c) metastasis from mucin secreting colonic carcinoma (H&E, 20X)
Discussion
Lesions of the urinary bladder, irrespective of their malignant status are responsible for significant morbidity and mortality. Cystoscopy is a primary diagnostic tool which allows a direct visualisation of the bladder mucosa helps in localising bladder tumours and facilitates biopsies from the suspected lesions [14]. For a definitive diagnosis histopathologic examination is mandatory. The role of the pathologist is not just limited to giving a diagnostic label, but also to giving additional information that can have an impact on the treatment [15].
Authors encountered quite a few lesions like cystitis cystica, cystitis glandularis and endometriosis which simulated malignancy on cystoscopy turning to be non neoplastic conditions on histopathology. There was a single case of high grade papillary urothelial neoplasm with extensive involvement of bladder mucosa but we could not demonstrate any microscopic features of muscle invasion to categorise it as invasive neoplasm even after sampling the entire specimen. These instances highlight the unique role of histopathological study not only supplementary but also proving to be crucial in the management of patients.
Hence, the present study was carried out with an aim to study the histopathological features of various lesions in the urinary bladder biopsies and to correlate the same with age, sex and other demographic characteristics. A total of 742 urinary bladder biopsy specimens were evaluated in our study. Majority were above 60 years of age with mean age of 59 years and sex ratio of 6.9:1.
The common age group affected according to our study was between 61-80 years constituting 57.81% of the cases and this was in concordance with Vaidya S et al., and Matalka I et al., [16,17]. The bladder lesions were more common in males with M:F ratio 7.6:1. It has been observed that there is wide range of M:F ratio observed between various studies ranging from 2.29:1 in study by Shah PY et al., to highest observed in study by Srikousthubha et al., [18,19].
Similar to the study of Gupta P et al., haematuria, was the most common complaint (99.5%) [20]. Personal habits like smoking, alcoholism and tobacco intake were seen in 549 (73.98%), 163 (21.96%) and 30 (4.04%) patients, respectively in the present study. These findings are comparable to the study conducted by Gupta P et al., the higher proportions of smokers in our study, may be due to the dominance of males [20]. Out of 46 non neoplastic lesions in the present study, 17 cases were chronic non specific cystitis and nine cases of other forms of cystitis like, polypoidal, follicular and eosinophilic cystitis were noted. These results were similar to study done by Srikousthubha et al., and Shruthi HP and Rangaswamy R, with chronic non specific cystitis as predominant type studied [19,21]. Other than the usual encounters like cystitis glandularis and cystitis cystica we noted two cases of diverticulitis and a case of endometriosis.
In the present study, one case of Von Brunn’s nest with cystitis cystica was noticed in a 42-year-old female, in the region of trigone, in which the urothelium showed a solid invagination into the lamina propria.
Among the neoplastic lesions papillary urothelial neoplasm was the most common one 678 cases (98.54.%), with majority of the cases were low grade non invasive papillary urothelial neoplasm, 332 (48.25%) cases. High grade non invasive papillary urothelial neoplasm 166 (24.12%) cases and invasive urothelial carcinoma 149 cases (21.65%). Rest 31 cases (4.57%) comprises insitu carcinoma, Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP), urothelial papilloma, inverted papilloma and nephrogenic adenoma.
Of the 149 invasive urothelial carcinomas studied, the predominant type 127 (85.23%) were invasive papillary urothelial carcinomas without any differentiation. A 21 cases (14.58%) invasive papillary urothelial carcinomas showed squamous differentiation and 1 case (0.69%) with glandular differentiation which was similar to studies done by Shruthi HP and Rangaswamy R, having 86.67% and Goyal VK et al., having 92.13% [21,22].
Other variants of Invasive urothelial carcinomas noted in the present study included, two cases each of microcystic and clear cell carcinoma (1.34%) each and one case of sarcomatoid carcinoma (0.69%). Out of 149 cases of invasive urothelial neoplasms in the present study, 3 (2.01%) were showing invasion in to lamina propria (superficially invasive bladder carcinoma) and 42 cases (28.18%) showing invasion in to superficial muscle layer and 102 (68.45%) cases showing invasion into deep muscle layer and 2 (1.34%) cases shows serosal penetration. Among different malignant lesions, urothelial carcinomas were followed by 2 cases each of (0.29%) urachal carcinoma and neuroendocrine tumours and 1 case (0.14%) of pure squamous cell carcinoma which is in accordance with Goyal VK et al., (93%) and discordance with Muhammed M et al., (93.3%) and Mahesh K and Yelikar BR, [22-24].
Urachal carcinomas accounts for 10% of all adenocarcinomas of the bladder [25]. Both patients of urachal carcinoma were thoroughly investigated but could not identify primary site elsewhere. In the present study, one case was well-differentiated non-bilharzial invasive SCC. According to recent study of the SEER database by Scosyrev E et al., SCC was more aggressive than papillary urothelial cancer after adjusting for common prognostic factors, such as stage [26]. Neuroendocrine tumours are very rare neoplasms in urinary bladder we had two cases one of which was paraganglioma. Among the urinary bladder tumours, paraganglioma is very rare and accounts for <0.06%. It arises from chromaffin tissue of the sympathetic nervous system within the layers of the bladder wall [27]. Small cell carcinoma showed serosal penetration and was positive for immunhistochemical markers for neuroendocrine differentiation.
Of 5 cases (0.67%) mesenchymal neoplasms, 2 cases (40%) were leiomyosarcoma, 2 cases (40%) of inflammatory myofibroblastic tumour and a single case of (20%) of leiomyoma. Leiomyosarcoma was seen more in elderly age group when compared with inflammatory myofibroblastic tumour and leiomyoma. Mesenchymal neoplasms were more common in females.
Mesenchymal neoplasms are very rare lesions in urinary bladder and the literature is growing with isolated case reports and short series, and the majority of cases reported are benign neoplasms. Leiomyosarcoma is the most common sarcoma seen in adults [28]. Eight cases were metastatic malignancy to urinary bladder with gastrointestinal tract being the most common primary site in 6 cases (75%), one case was a prostatic metastasis to bladder and other was from invasive lobular carcinoma breast (12.5% each). Metastasis from solid tumours to urinary bladder is very rare and represents 2% of all bladder neoplasms. Breast cancer as a primary site is seen in about 2.5% cases of all metastatic bladder cancer [29]. Secondary adenocarcinoma involves the bladder either by direct extension or by metastasis from a distant site. The common primary sites include the colon, prostate, endometrium, cervix, breast, and lung [30].
In the present study, six secondary adenocarcinomas were found, four were known cases of carcinoma colon, two were known cases of carcinoma rectum one case were lobular carcinoma metastasis and one was direct invasion from prostaticadenocarcinoma. Diagnosis was made after considering the clinicoradiological findings, raised PSA levels, and IHC markers. In the current study, authors were emphasising more on the histopathological diagnosis with respect to histological grading of bladder tumours. Authors followed, World Health Organisation (WHO)/International Society of Urologic Pathologists (ISUP) (2016) classification for histological grading in the present study which has the advantages of uniform terminology, well defined criteria for non neoplastic and neoplastic bladder tumours leading to greater interobserver reproducibility [12].
Limitation(s)
In the present study, authors concentrated mainly on the histomorphological features and hence could not contribute much to the natural history and pathogenesis of the disease in the patients.
Conclusion(s)
To exclude the discrepancies between cystoscopic observations, particularly in tumefactive non neoplastic diseases histopathologic confirmation is very essential. Since grading, invasiveness and histological categorisation have great importance in therapeutic and prognostic point of view; histopathological examination is considered as a gold standard in evaluation of bladder biopsy in urological diseases by comparing the clinical diagnosis and histological observations.
PUNLMP: Papillary urothelial neoplasm of low malignant potential
HGPUC: High grade papillary urothelial carcinoma; LGPUC: Low grade papillary urothelial carcinoma; PUNLMP: Papillary urothelial neoplasm of low malignant potential