Breast cancer is the second leading cancer in India, according to global disease burden 2016 study reports [1]. Histopathology is quintessential in grading and staging of breast cancer and also in establishing an effective therapeutic management of patients with breast cancer [2]. The routine conventional processing involves a multitude of chemical solutions in different concentrations and takes 14-16 hours both in histokinette and manual processing. The alternatives available are cryostat with freeze fixation and laboratory microwave with special fixatives. The last few decades have seen the introduction of microwaves in the histopathology laboratory for a wide range of applications. Routine conventional processing has been the most commonly used method until now. The main advantage being its reliability whereas disadvantage is that it is labour intensive and time consuming taking about 16-18 hours [3].
In microwave assisted technology, the heat waves produced aid in effective diffusion of fluids in tissue blocks or sections in a considerable shorter time span compared to conventional heating [3-5]. Microwave induced heat reduces the poor conduction of heat in tissues [6]. Unlike conventional heating, the effect occurs simultaneously throughout the whole material processed in microwave (‘internal heating’) [3]. This has helped in reducing the turnaround time, which could only be possible because a lower time was required for histoprocessing, and this has been effective enough to provide the diagnosis on the same day for a variety of tissue biopsy specimens.
With recent improvements, the microwave assisted tissue processing is becoming more widely accepted in the diagnostic surgical pathology laboratory therefore the application of various diagnostic techniques to microwave processed tissues will also be increasing. The use of microwave processed tissue has yielded positive results in several studies [7-10]. However, there exists a concern among surgical pathologists that microwave processing may alter the quality, sensitivity and specificity of immunohistochemical staining of the various antibodies used in the surgical pathology laboratory [11].
Staining technique is an integral part of histopathological processing and the quality of staining impacts the interpretation. The efficacy of staining is dependent on multiple parameters and heat energy is one of the most important factors [12]. The aim of the present study was to compare the quality of immunohistochemical staining of microwave and conventional tissue processing methods by evaluating the ER and PR antigens on paired breast cancer samples. The objective of the study was to evaluate sensitivity, specificity, PPV and NPV of ER and PR expression on microwave processed specimens. Another objective of the study was to compare ER and PR expression in carcinoma breast after conventional and microwave fixation.
Materials and Methods
A cross-sectional study was conducted from February 2014 to September 2015 in the Department of Pathology at a tertiary care centre in Southern India. Ethical approval was obtained from the Institutional Ethics Committee (IEC no. IEC/MES/13/2014)
Inclusion criteria: All the Fine Needle Aspiration Cytology (FNAC) and radiologically diagnosed samples of breast carcinoma patients who underwent true cut biopsy, lumpectomy and mastectomy were considered for evaluation of ER and PR status and were included in the study.
Exclusion criteria: Degenerated, over-fixed, outside processed samples and inadequate true cut biopsy samples were excluded from the study in order to maintain the purity and uniformity of the samples.
Study Procedure
A total of 176 tissue blocks were used for the preparation of 44 paired samples, all of which pertained to invasive carcinoma of breast. The specimens were fixed in formalin for a minimum of six hours. For lumpectomy and mastectomy specimens, sections were taken which were of 1.5-2 mm in thickness and measuring not more than 3 cm. Each section was equally divided and placed in plastic cassettes. One from each pair to be processed by different methods was processed with a commercially available domestic microwave and the other was processed with the routine conventional processing using the Histokinette.
A routine domestic cooking microwave oven (IFB microwave appliance, Model no: 23BC3 Input-1400 W, Output-900 W) was used for the histopathology processing in present study. A pilot exercise was done using 10 tissue samples and the baseline timings and pressure were standardised to attain a temperature of 60-62°C. Plastic cassettes containing the tissue sections were placed in 500 mL microwave compatible containers at equal distance from one another to prevent overlapping that could hamper the diffusion of the solution into the tissues. The power mode in the microwave oven was adjusted from high to low. The volume of each reagent was taken in such a way to just immerse the tissue cassettes. To ensure adequate fixation, the samples were processed in microwave for 4 minutes in 10% formalin and kept for 30 minutes at room temperature.
Samples were rinsed in absolute alcohol. Dehydration was done with 95%, 95% and 100% ethyl alcohol at optimised pressure and time ((20 P/9″, 20 P/9″, 50 P/4″) respectively. The first solution of alcohol used each time was fresh. Clearing was done with three changes of absolute alcohol at 50 P, four minutes each. The cassettes were transferred into a beaker containing 300 mL of molten paraffin wax preheated at a temperature of 70°C. Wax impregnation with two changes of paraffin wax at 50 P for one and three minutes each.
For the conventional processing, the specimens were kept in 10% formalin for overnight fixation, followed by routine conventional tissue processing using Leica bio systems automated tissue processor Histokinette. About 3 μ section thickness was taken using a Leica rotary microtome for consequent H&E staining [Table/Fig-1].
Protocol used for microwave processing and conventional processing.
| Microwave processing | Conventional tissue processing in Histokinette |
|---|
| Steps | Reagent | Pressure (P) | Time (minutes) | Reagent | Time (minutes) |
|---|
| Fixation | 10% formalin | 50 | 4 | 10% formalin | 60 |
| Keep for 30 minutes | | 30 |
| 10% formalin | 50 | 4 | 10% formalin | 60 |
| Rinse in absolute alcohol | | |
| Dehydration | 95% IPOH | 20 | 5 | 70% IPOH | 60 |
| 95% IPOH | 20 | 5 | 80 % IPOH | 60 |
| 90% IPOH | 60 |
| 100% IPOH | 50 | 2 | 95% IPOH | 60 |
| Keep for 30 minutes | | 30 | 100% IPOH×2 | 120 |
| Cleaning | 100% IPOH | 50 | 2 | Xylene | 60 |
| 100% IPOH | 50 | 2 | Xylene | 60 |
| 100% IPOH | 50 | 2 | | |
| Impregnation | Melted paraffin wax | 50 | 1 | Paraffin wax | 180 |
| Keep for 10 minutes | | 1 | Paraffin wax | 180 |
| Melted paraffin wax | 50 | 3 | | |
| Total time consumed | | 92 | | 960 |
*IPOH: Isopropyl alcohol; P: Pascals
The slides were H&E stained, cover slipped, and evaluated for diagnosis by a dedicated pathologist. The microwave processing took a total time of 92 minutes, whereas the conventional processing took 16 hours. 4 μm sections were cut from tissue blocks and then placed on pretreated glass slides, then kept at 60°C for 60 minutes. Sections were de-waxed and rehydrated with graded alcohol. Immunostaining of the paired tissues was done and evaluated with controls for each paired sample. The ER and PR hormone receptor assessment was done based on the percentage of tumour cells staining positively and the intensity of staining, which was graded as low, moderate or strong was reported.
The slides were scored with Allred scoring system for intensity and proportion by three pathologists independently in order to maintain accuracy and prevent observational bias. The criterion for considering a case as positive was a minimum of 1% of tumour cells positive for ER or PR. The American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) guidelines for hormone receptors in breast cancer and the Allred scoring system were used [Table/Fig-2] [13].
Allred scoring system* used for Estrogen and Progesterone Receptor (ER and PR) evaluation.
| Positive cells (%) | Proportion score | Intensity | Intensity score |
|---|
| 0 | 0 | None | 0 |
| <1 | 1 | Weak | 1 |
| 1-10 | 2 | Intermediate | 2 |
| 11-33 | 3 | Strong | 3 |
| 34-66 | 4 | | |
| ≥67 | 5 | | |
*The Allred Score is a combination of the percentage of positive cells and the intensity yielding a score of 8. A score of 0 and 2 denotes negative scoring and a score of 3-8 are considered positive
Statistical Analysis
The data were entered in Microsoft Excel 2017 and Epi Info software (Version 7) was used for further analysis. Validity of ER, PR receptor status was evaluated with sensitivity, specificity, PPV and NPV. Kappa statistics and Spearman’s rank correlation test was used to denote the agreement between ER and PR hormone receptor status.
Results
A higher proportion of patients with breast carcinoma were in the age group of 45-80 years (32/44, 72.8%) in the present study. Youngest patient was 28-year-old and the oldest patient was 80-year-old. The mean age was 51.32±11.16 years [Table/Fig-3]. All the 44 paired samples included in the study were diagnosed as invasive carcinoma of breast of no special type.
| Procedure | Age (years) | Frequency (n) |
|---|
| 25-34 | 35-44 | 45-54 | 55-59 | >60 |
|---|
| Mastectomy | 1 | 4 | 11 | 4 | 6 | 26 |
| True cut biopsy | 1 | 5 | 5 | 2 | 4 | 17 |
| Lumpectomy | 0 | 1 | 0 | 0 | 0 | 1 |
| Total (%) | 2 (4.54) | 10 (22.73) | 16 (36.4) | 6 (13.6) | 10 (22.73) | 44 (100) |
The ER status evaluation on microwave processed samples, showed a sensitivity of 87.50%, specificity of 100%, PPV of 100% and NPV of 93.3%. Cohen’s Kappa statistics for dichotomised results showed that there was an almost perfect agreement in the results of the paired samples k=0.902 (p<0.001). Spearman’s rank correlation analysis was performed for scoring the percentage of positive cells and intensity [Table/Fig-4a,b]. Validity of PR status evaluation showed a sensitivity of 92.90%, specificity of 100%, PPV of 100% and NPV of 96.77% [Table/Fig-5]. Substantial agreement between paired samples was observed with r=0.904 (p<0.001) [Table/Fig-6]. Kappa statistics for dichotomised results for PR status showed that there was an almost perfect agreement in the results of the paired samples k=0.951 (p<0.001). Spearman’s correlation analysis showed a strong association between the paired samples with r=0.975 (p<0.001) [Table/Fig-7]. [Table/Fig-8a,b] illustrate the percentage of positive cells and intensity accounted for the Allred scoring system.
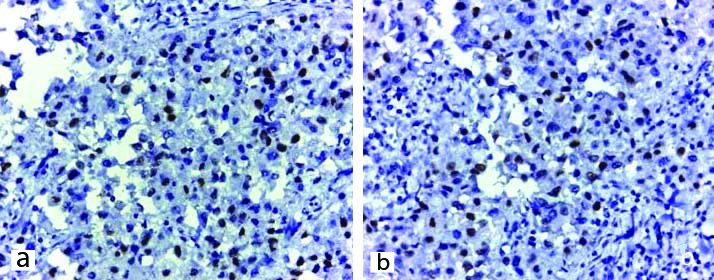
a) Photomicrograph showing ER positivity of Microwave processed paired sample, Allred Score-6 (40X); b) Photomicrograph showing ER positivity of conventionally processed paired sample, Allred Score-6 (40X).
Validity of immunohistochemical staining of oestrogen and progesterone hormone receptors in microwave processing keeping conventional tissue processing as Gold Standard
| Parameter | ER (n=44) | PR (n=44) |
|---|
| Sensitivity | 87.50% | 92.90% |
| Specificity | 100% | 100% |
| Positive Predictive Value (PPV) | 100% | 100% |
| Negative Predictive Value (NPV) | 93.3% | 96.77% |
*ER: Estrogen receptor; PR: Progesterone receptor
Measure of agreement and Spearman’s correlation coefficient of microwave and conventional tissue processed ER values.
| Parameters | ER (n=44) |
|---|
| Correlation | Spearman’s correlation coefficient | 0.904 (p<0.001) |
| Measure of agreement | Kappa | 0.902 (p<0.001) |
Measure of agreement and Spearman’s correlation coefficient of microwave and conventional tissue processed PR values.
| Parameters | PR (n=44) |
|---|
| Correlation | Spearman’s correlation coefficient | 0.975 (p<0.001) |
| Measure of agreement | Kappa | 0.951 (p<0.001) |
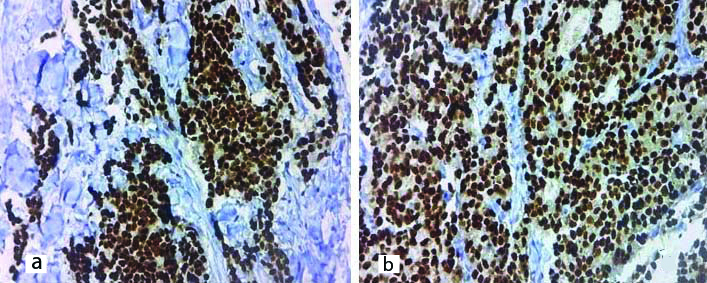
a) Photomicrograph showing PR positivity of microwave processed paired sample, Allred Score-8 (40X); b) Photomicrograph showing PR positivity of conventionally processed paired sample, Allred Score-8 (40X).
Discussion
Conventional tissue processing technique has been widely used for several decades; however the procedure is prolonged and a laborious procedure. A major disadvantage of this method is the use of harmful chemicals like xylene and formalin. In present study, authors have used formalin as fixative but use of xylene for clearing was eliminated and replaced by isopropyl alcohol [14]. The antigen retrieval for immunostaining was done with a pressure cooker. The present study group constituted patients from the third to sixth decade. All the 44 cases were diagnosed as invasive carcinoma breast of no special type. No cases of lobular carcinoma or other special or mixed types were diagnosed in the present study. According to published series invasive carcinoma of breast no special type constituted to 40-70% of the cases studied [15-17].
In the present study, there were 44 cases on which ER and PR was evaluated. Our experience from prior studies advocates the use of domestic microwave oven. The total processing time was 111 minutes when 500 mL containers were used according to Kok LP and Boon ME [5]. Throughout the steps, a working temperature of 75°C was maintained. In this study, the total time taken for the entire microwave processing was 92 minutes for 500 mL capacity. It was observed that any temperature above 70°C was causing the isopropyl alcohol to be opacified and so in the present study, the temperature was maintained at 62°C for all steps. An advantage to this was that the isopropyl alcohol could be reused. Although it was not possible to assess the temperature for the core biopsies, the required temperature was maintained. The major fluctuations in voltage were minimised with the use of a stabiliser.
In the H&E sections, no significant difference was observed between the nuclear size and shapes and the staining characteristics were discernible. This was in accordance with other concomitant studies [9,18]. Observations were made with regard to technical aspects and cell morphology on H&E staining. Tissue adherence was found to be reduced during the immunostaining processing in the microwave processed tissue sample. However, only one conventionally processed sample showed a technical difficulty in tissue adherence during the immunostaining process, whereas five of the microwave processed specimens showed reduction in the tissue adherence. This was similar to the study conducted by Emerson LL et al., where this difference in section adherence during the immunostaining process was attributed to the variability among the various microwave processors in the amount of heat produced and the high energy processor which was used [19]. With the advent of newer versions of microwave which are commercially available, limitations in tissue processing technique imposed by fluctuation in voltage and temperature are controlled effectively. However, such microwaves are much costlier compared to the domestic microwaves which are readily available and affordable. These domestic microwaves have been used earlier for tissue processing.
Both the microwave processed, and the conventionally processed tissue did not showed any significant difference in the quality of staining by H&E method. This finding was similar to the reports of several studies [20-26]. In this study, it was observed that the slides of microwave processed tissue showed brighter staining with eosin as compared to the conventionally processed sections. Similar findings were observed in studies where eosinophilia in tissues fixed by microwave were independent of the solutions used for tissue processing [27,28]. Reports by Leong AS et al., concluded that the cytoplasm of eosinophilia produced greater enhancement of the nuclear cytoplasm contrast [25]. They also suggested that the eosinophilia staining brightness maybe corrected by altering the staining time in eosin staining.
Few other observations made were the slight condensation of stroma focally similar to a other reports [29]. Focal condensation of connective tissue is not much significant in diagnostic pathology, as explained by Kok LP et al., [30]. Also, in the present study, the Red Blood Cells (RBC) were found to be preserved. There exists ambiguity in this observation across the published literature with a few authors reporting preserved RBC whereas other found them to be lysed [9].
The cut-off for ER immunostain positivity was kept at one percent since it has been clinically validated as a predicting response to tamoxifen based therapy [6]. Allred score of three or more (based on definition of ER positivity) corresponds to as few as 1% of cells showing weak immunostaining signal. Out of the paired samples processed by microwave irradiation, applying the dichotomised results 14 (31.8%) cases were evaluated as positive, and 30 (68.2%) were labelled ER negative. Kappa statistics for dichotomised results showed that there was an almost perfect agreement in the results of the paired samples k=0.902. Out of the positive cases for both the paired samples, the staining of ER showed similar results for both the microwave processed and the conventionally processed samples. Emerson LL et al., in his study of immunohistochemical stain quality on conventional and rapid microwave processed tissues using tissue array found that there is a very high concordance between intensity and extent of immunostaining in both processing methods. They also reported that the antigen retrieval was enhanced by microwave processing; this improves the sensitivity of many antibodies in the field of clinical practice [19] whereas, it has also been observed that in certain clinical situations antigen retrieval would be detrimental to the specificity and to the clinical uses of immunostaining as in c-kit.
Reports from Pegolo E et al., study revealed a perfect agreement between the paired tissues when evaluating the ER status. The entire microwave processed paired samples of the ER positive cases with the conventional processing were also positive for the same [31]. The intensity of the immunohistochemical reaction was also observed to be similar. A few authors have reported that when a dichotomised score (positive/negative) was used the ER reaction in both neoplastic cells and non neoplastic epithelium in formalin-fixed tissue and molecular fixative-exposed specimens were comparable [32,33].
Cut-off positivity for PR immunostain positivity was also similar to that of ER, which was one percent as it has been clinically validated as predicting response to tamoxifen-based therapy. Allred score of three or more (i.e., definition of ER positivity) corresponds to as few as 1% of cells showing weak immunostaining signal.
Out of the 44 cases in the present study, 14 (31.8%) samples processed by the conventional method were evaluated as ER positive and 30 (68.2%) cases as ER negative. Out of the paired samples processed by microwave irradiation, 13 (29.5%) cases were evaluated as positive, and 31 cases (70.5%) were labelled ER negative. Out of the positive cases for both the paired samples, the staining of PR was observed to be identical for both the microwave processed and the conventionally processed samples.
Out of the positive cases for both the paired samples, the staining of ER was observed to be identical for both the microwave processed and the conventionally processed samples. This was in accordance with the study by Pegolo E et al., which reported perfect agreement between the paired tissues when evaluating PR status (k=1) [31]. The study also reported that the intensity of the immunohistochemical reactions for PR was found to be similar. All the PR positive samples with the conventional processing were also positive with the microwave processing. The intensity of the immunohistochemical reaction was also observed to be similar.
Limitation(s)
One limitation observed was that although a domestic cooking microwave with stabiliser to control major variations in temperature and voltage was used, there still seemed to be some variation which may have caused the reduced tissue adherence during immunostaining in some of the microwave processed samples.
Conclusion(s)
Microwave aided tissue processing has two significant advantages over conventional method being less laborious and aids in rapid diagnosis. The overall qualities of staining of tissue sections were similar in conventional tissue processing and microwave assisted tissue processing techniques which make microwave processing a better technique.
Hence, results of the study are in support to the growing evidence that microwave tissue processing is fast, reliable and cost effective and it can replace conventional processing in the field of immunohistochemistry for faster results.
*ER: Estrogen receptor; PR: Progesterone receptor