Aging is allied with a variety of functional, demographic and immunologic changes, that accounts to increase in incidence and severity of infectious diseases among the elderly [1,2]. Immunoscence is the alteration of immune system due to ageing and is persistent among all elderly in varying degrees and there is a link between the degree of frailty and immunocompetency [3]. As many of the elderly are on multiple medications for underlying illnesses, thus management is complicated due to age-related organ system changes, therefore antimicrobial therapy needs to be chosen keeping drug interactions and adverse events in mind [1-4]. Vitamin D is one potent antimicrobial agent [5].
Vitamin D is responsible for regulation of more than 200 genes, including cell proliferation, differentiation and apoptotic genes [6]. It acts as the key holder for modulating systemic inflammation, oxidative stress and mitochondrial respiratory functions. Thus a vitamin D repletes state appears to benefit most infections [7]. Adjunctive treatment of vitamin D against different infection is coming in focus today [8,9]. Vitamin D deficiency has been found to be prevalent among rural elderly women in West Bengal [10]. Besides that there is no such research work conducted among elderly women specially residing at rural area of India. Hence, the objective of the present study was to examine the association of vitamin D status with antimicrobial activity of cultured macrophages isolated with in an exclusively elderly population cohort. Also intended to determine the antimicrobial activity of cultured macrophages after in-vitro supplementation of 1,25 Dihydroxy Vitamin D.
Materials and Methods
This experimental study was conducted among elderly women from 30 villages of Amdanga block, North 24th Parganas district, West Bengal, India, with in the time period of April 2014 to August 2018. Ethics clearance was obtained from the Institutional Ethics Committee of All India Institute of Hygiene and Public Health (AIIH&PH), Kolkata. Informed written consent was obtained from each study subjects prior to the commencement of the study.
Inclusion criteria: Healthy elderly women aged between 60-70 years, who were co-operative in nature and willing to participate were included in the study.
Exclusion criteria: Elderly women having previous history of thyroid dysfunction, on hormonal replacement therapy, amenorrhoea due to any pathological cause or surgery, on vitamin D supplementation and physically or mentally challenged were excluded from the study. Elderly women having fever in last 20 days and having high total White Blood Cells (WBC) count (4.5-11.0×109/L [11]) and high C-reactive protein level (below 1 mg/L [12]) were also excluded from the study.
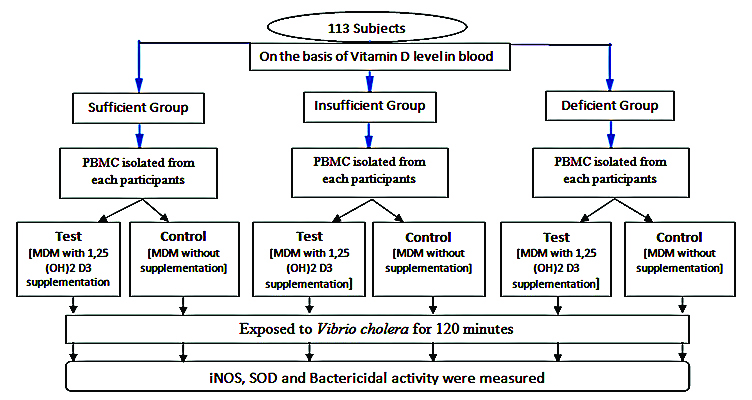
Sample size: Total 113 elderly women were selected randomly with in the study period of April 2014 to August 2018.
Data Collection
At first stage the elderly were selected according to their serum 25(OH)D level and classified into three groups [13] i.e.,
Sufficient group (≥30 ng/mL) -60 subjects
Insufficient group (21-29 ng/mL) -27 subjects
Deficient group (≤20 ng/mL) -26 subjects
In the next stage the Peripheral Blood Mononuclear Cells (PBMC) were isolated from fresh blood (4 mL) of each of the studied subjects and the collected PBMC were divided into two groups i.e., test and control; where test group received vitamin D supplementation {1, 25(OH)2D was supplemented in liquid form by mixing it gently with the given culture medium (RPMI-1640)} and control groups did not receive any supplementation. Finally, after the development of Monocyte Derived Macrophages (MDMs), each test and control group were exposed to Vibrio cholerae infection for 120 minutes.
Isolation and Culture of Human Macrophages
PBMC were isolated from heparinised blood (4 mL) of each and every study subjects by density gradient centrifugation with Ficoll-Paque [14]. The cells were washed twice in Phosphate-Buffered Saline (PBS) and were resuspended in medium RPMI 1640 (HIMEDIA), supplemented with 10% Fetal Calf Serum and Macrophage Cell Stimulating Factor (MCSF) also added at 2 ng/mL concentration. Finally, cells were added to adherent 6 well plates at a density of 2×106 cells per well. After incubation for 48 hours, at 37oC and 5% CO2 environment, the non-adherent cells were removed by repeated vigorous washings. Selected cell culture was then supplemented with 1,25(OH)2D at a dose of 10×10-8 M for 72 hours. The dose was standardised and referred by previous reports [15-18]. After completion of seven days the cells were isolated and incubated with V. cholerae for 120 minutes. The infections were given in ratio of 1:40. After which inducible nitric oxide synthase (iNOS) activity and Superoxide Dismutase (SOD) activity and bacteria killing assay were performed [Table/Fig-1].
Antimicrobial Activity Assessment
The following assays were done following standard methods.
Inducible Nitric Oxide Synthase (iNOS) Activity Assay [19]
Macrophage cell lysate from individual subjects were assayed for iNOS enzyme using a reaction system (arginine as substrate, potassium ferrocyanide and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer) and observed spectrophotometrically at 420 nm. Enzyme activity was expressed as ∆OD/mg protein per unit time.
Super Oxide Dismutase (SOD) Activity Assay [20]
Macrophage cell lysate from individual subjects were assayed for SOD enzyme using a reaction system where pyrogallol was used as substrate and observed spectrophotometrically at 420 nm. The enzyme activity was expressed as ∆OD /mg protein per unit time.
Total protein estimation [21]
The protein contents of the samples were quantified by standard method [21]. The absorbance was measured at 660 nm.
Bacteria Killing Assay [22]
Human MDMs (2×106 cells) were suspended at 1:40 ratio with Vibrio cholerae in a final volume of 1mL of 100 mM Phosphate buffer, pH 7.4. This suspension was then incubated with gentle rocking, at 37oC. Centrifugation followed by washing with cold buffer twice. Cells were bursts by freeze-thaw method. Aliquots of that suspension were plated at zero minute, and 120 minutes for incubation at 37oC on nutrient media [Table/Fig-2]. The Agar plates were then incubated at 37oC in incubator, and bacterial colonies were counted on the next day. Results were expressed as bacterial killing=100-(N/N0 multiplied by 100) where N is the number of colonies counted at each time point and N0 is the number of colonies counted at time zero.
Bacteria killing assay for Vibrio cholerae (Nutrient agar plate).
Statistical Analysis
Data were put in Microsoft Excel Worksheet (Microsoft, Redwoods, WA, USA) and they were checked for accuracy. Continuous data was first checked for normality distribution by Kolmogarov Smirnov Test. Significant p-value indicated skewed distribution. In presence of skewed distribution, non parametric tests were performed. Difference between distributions of continuous dataset from three different independent groups was determined by Kruskall-Wallis test. Correlation was calculated by Spearman’s correlation coefficient (ρ, rho). Correlation was calculated by Spearman’s correlation coefficient (ρ, rho). SPSS software, version 20.0 (Statistical Package for the Social Sciences Inc, Chicago, IL, USA) was used to perform statistical analysis. The p-value equal to or less than 0.05 was considered as statistically significant.
Results
Association of serum 25(OH)D status with bacteria killing assay, iNOS and SOD activity
As per bacteria killing assay the sufficient group has significantly (p-value=0.008) higher CFU reduction rate than the other two groups. After in-vitro 1, 25(OH)2D supplementation there is no significant differences observed in bacteria killing assay (p-value=0.58).
In conditions, where there is no exposure to infection or 1, 25(OH)2D treatment the median iNOS level was found to be more or less similar in three different group of serum 25(OH)D level. After exposure to infections the median iNOS level was found to be 2.27 in deficient group then median level increases to 3.47 among insufficient group, again it decreases to 2.52 in sufficient group. Distribution of these values in three different group was found to be statistically significant as evident from Kruskall-Wallis test (p-value=0.005). Again with increase in serum 25(OH)D level, the iNOS level decreased significantly (Spearman’s, rho p=0.04). In conditions where 1, 25(OH)2D were treated with exposure to infection the median iNOS level was very low in deficient group 2.93 followed by increase in insufficient (4.19) group and again decreases in the sufficient group (3.52). Although the values of iNOS level was not statistically significant in three different groups (p-value=0.14) [Table/Fig-3].
Inducible Nitric Oxide Synthase (iNOS) and Superoxide Dismutase (SOD) activity in-vitro cultured human macrophages of elderly women according to 25(OH)D level in comparison to those with or without exposure to infection (V. cholerae) (N=113).
| Serum 25(OH)D status | iNOS level | Kruskal-Wallis statistic (p value) and Spearman’s rho (p-value) | SOD level | Kruskal-Wallis statistic (p value) and Spearman’s correlation rho (p-value) |
|---|
| Median (IQR) | Median (IQR) |
|---|
| Without infection |
| Deficient group | 2.12 (1.25) | 0.554 (0.757)-0.047 (0.615) | 3.42 (3.06) | 1.76 (0.4135)-0.0007(0.993) |
| Insufficient group | 2.19 (1.70) | 3.49 (2.31) |
| Sufficient group | 2.13 (2.18) | 2.84 (2.28) |
| With Infection (V.cholerae) |
| Deficient group | 2.27 (1.87) | 10.4 (0.005)-0.188 (0.045) | 4.41 (4.45) | 2.65(0.2657)-0.120(0.2036) |
| Insufficient group | 3.47 (2.88) | 4.89 (3.9) |
| Sufficient group | 2.52 (2.25) | 3.23 (3.87) |
| With Infection(V.cholerae) + 1, 25(OH)2D treatment |
| Deficient group | 2.93 (3.47) | 3.89 (0.142)-0.088 (0.350 | 3.84(3.4) | 2.42(0.297)0.037(0.6968) |
| Insufficient group | 4.19 (5.31) | 5.2 (4.95) |
| Sufficient group | 3.52 (3.41) | 4.6 (6.26) |
In conditions, where there is no exposure to infection or 1, 25(OH)2D treatment the median SOD level was 3.42 for deficient group and 3.49 in case of insufficient group, 2.84 in case of sufficient group. Moreover, the difference between these three groups were insignificant (p-value=0.41) as revealed from Kruskal-Wallis test [Table/Fig-3].
Antimicrobial effects after in-vitro 1, 25(OH)2D supplementation
In bacteria killing assay after in-vitro 1, 25(OH)2D supplementation, the CFU reduction rate were (N=10) 24.16%, (N=10) 25.35% and (N=10) 35.59% among sufficient, insufficient and deficient group. Where without supplementation the CFU reduction rate were 43.40% (N=10), 16.31% (N=10), and 15.10% (N=10) respectively. It has been observed that the increase in CFU reduction rate was not significant for sufficient group (p-value=0.22), insufficient group (p-value=0.09) and deficient group (p-value=0.17) respectively. Similarly, overall CFU reduction rate after in-vitro 1, 25(OH)2D supplementation was insignificant (p-value=0.44).
In conditions, where there is no exposure to infection the iNOS level of the deficient group was 2.12, with exposure to infection (V. cholerae) it increases 2.27 and after in-vitro 1, 25(OH)2D supplementation and with exposure to infection (V. cholerae) the iNOS activity level increases to 2.93, which was significant as evident from Kruskal-Wallis test (p-value=0.01). Considering SOD activity among deficient group no such significant increase was observed.
Whereas in case of insufficient group when there is no exposure to infection the iNOS level of the insufficient group was 2.19, with exposure to infection (V. cholerae) it increased to 3.47 and after in-vitro 1, 25(OH)2D supplementation and with exposure to infection (V. cholerae) the iNOS activity level increased to 4.19, which was significant from Kruskal-Wallis test (p-value=0.002). Considering SOD activity among the insufficient group, increase in SOD activity were statistically significant (p-value=0.04) as per Kruskal-Wallis test [Table/Fig-4].
Inducible nitric oxide synthase (iNOS) and Superoxide Dismutase (SOD) activity in-vitro cultured human macrophages of elderly women according to 25(OH)D level in comparison to those with or without exposure to infection (V.cholerae) (N=113).
| Serum 25(OH)D status | iNOS level | Statistical test | SOD level | Statistical test | 25(OH)D level |
|---|
| Median (IQR) | Kruskal-Wallis statistic (p value) | Median (IQR) | Kruskal-Wallis statistic (p value) | Median (IQR) |
|---|
| Deficient group (N=27) |
| Without infection | 2.12 (1.25) | 9.20 (0.011) | 3.42 (3.06) | 2.12 (0.346) | 14.34 (10.68) |
| With Infection (V.cholerae) | 2.27 (1.87) | 4.1 (4.45) |
| With Infection (V.cholerae)+1, 25(OH)2D treatment | 2.93 (3.47) | 3.84 (3.40) |
| Insufficient group (N=26) |
| Without infection | 2.19 (1.70) | 20.09 (0.002) | 3.49 (2.31) | 6.38 (0.041) | 26.74 (4.31) |
| With Infection (V.cholerae) | 3.47 (2.88) | 4.89 (3.9) |
| With Infection (V.cholerae)+1, 25(OH)2D treatment | 4.19 (5.31) | 5.20 (4.95) |
| Sufficient group (N=60) |
| Without infection | 2.13 (2.18) | 8.92 (0.011) | 2.84 (2.28) | 18.65 (0.0001) | 43.69 (14.05) |
| With Infection (V.cholerae) | 2.52 (2.25) | 3.32 (3.87) |
| With Infection (V.cholerae)+1, 25(OH)2D treatment | 3.52 (3.41) | 4.6 (6.26) |
| Total (N=113) |
| Without infection | 2.12 (1.57) | 29.05 (0.001) | 3.25 2.49) | 22.95 (0.001) | 31.33 (24.58) |
| With Infection (V.cholerae) | 2.62 (2.3) | 3.77 (3.94) |
| With Infection (V.cholerae)+1, 25(OH)2D treatment | 3.40 (3.5) | 4.59 (4.25) |
Again in case of sufficient group when there was no exposure to infection, the iNOS level of the sufficient group was 2.13, with exposure to infection (V. cholerae) it increased to 2.52 and after in-vitro 1, 25(OH)2D supplementation and with exposure to infection (V. cholerae) the iNOS activity level further increased to 3.52, which was significant according to Kruskal-Wallis test (p=0.01). Considering SOD activity among sufficient group, again SOD activity increased significantly (p=0.001) as revealed from Kruskal-Wallis test.
Discussion
Macrophages are the primary professional scavenger cells. Capable to engulf micro-organisms, proteins and other smaller cells using several mechanisms such as Fc- receptor and complement mediated phagocytosis and endocytosis [23,24]. Vitamin D helps in boosting the activity of monocyte and macrophages, thereby contributing to a potent systemic anti-microbial effect [6-8]. Thus, vitamin D deficient/insufficient state is always beneficial to infections.
According to Chalmers JD et al., 25(OH)D deficiency is frequent in brochiectasis and has significant correlations with the markers of disease severity [25]. Zosky GR et al., proved the direct mechanistic evidence, linking between vitamin D deficiency and lung development, the association between obstructive lung disease and vitamin D status [26]. According to McCauley et al., higher 25(OH)D levels in children with cystic fibrosis were associated with lower rates of pulmonary exacerbations [27]. In present study, considering bactericidal activity (p-value=0.003), iNOS activity and SOD activity also showed the similar trend where sufficient serum 25(OH)D consisting group had better result than others.
iNOS is an essential enzyme in protective immunity against different bacterial infections [28]. Nitric oxide can inhibit both microbial DNA replication and cellular respiration [29]. Activated macrophages massively generate nitric oxide, NO and superoxide radicals [30]. In fact, the bactericidal effect of polymorphonuclear leucocytes depends on their superoxide generative capacity [27-30], and biosynthesis of SOD [31].
Previous reports showed that in-vitro vitamin D treatment enhanced the bacteria killing activity significantly [27,31,32]. In present study, in-vitro 1, 25(OH)2D supplementation increases iNOS and SOD activity significantly, although no significant impact was observed in CFU reduction rate among all three groups.
Present research is one basic approach towards understanding one promising molecular candidate to combat infections among elderly. It has many limitations as well. Therefore more elaborative and specific study should be conducted to find out a novel anti-infectious intervention, while many antibiotic classes have lost their antimicrobial efficacy and multidrug-resistance constitutes an emerging threat to global health.
Limitation(s)
The small sample size was one of the important limitations of the present study, additionally more elaborative and specific study might give more clear picture than the present one.
Conclusion(s)
Bacteria killing capacity of macrophages varies significantly with serum 25(OH)D level of the target population. Sufficient group’s macrophages always had better profile than other two groups. In-vitro 1, 25(OH)2D supplementation increases iNOS and SOD activity significantly.