The COPD is characterised by persistent airflow limitation which is a global health issue having high social and economic burden. Development of COPD is associated with chronic bronchial and alveolar inflammation in response to noxious particles or gases, primarily those in tobacco smoking exposure. The pathogenetic mechanisms behind these systemic effects are poorly understood but are probably interrelated and multifactorial, including systemic inflammation, physical inactivity, tissue hypoxia, and oxidative stress, among others. Extrapulmonary manifestations of COPD may inversely lead to worsened dyspnoea, impaired functional status, reduced health-related quality of life, and increased mortality of these patients [1].
The T2DM is a metabolic disorder affecting approximately 300 million individuals worldwide. Numerous studies have shown that low-grade chronic inflammation is part of the insulin resistance syndrome and is associated with the development of T2DM. Accordingly, chronic systemic inflammation is probably one of the common denominators between COPD and T2DM. Epidemiological studies have found that T2DM is more frequent in COPD patients and is likely to affect their prognosis. On the other hand, a series of studies have reported an association between DM and reduced lung function. Undoubtedly, the relationships between these two complex diseases are complicated, and more research into this issue is required to foster our understanding of them [1].
It is estimated that the world population will reach a record 7.3 billion and the high burden of chronic conditions associated with ageing and smoking will increase further. It is now consensually agreed that an estimated number of 328 million people have COPD worldwide that is 168 million men and 160 million women [2-7]. India also contributes a significant and growing percentage of COPD mortality which is estimated to be amongst the highest in the world; i.e., more than 64.7 estimated age standardised death rate per 100,000 amongst both sexes as mentioned in the World Health Organisation (WHO) Global Infobase Updated on 20th January 2011 (India 102.3 and China 131.5) [8]. This would translate into approximately 556,000 in case of India (>20%) and 1,354,000 cases in China (about 50%) out of a world total of 2,748,000 annually [9].
Diabetes is fast gaining the status of a potential epidemic in India with more than 62 million diabetic individuals currently diagnosed with the disease. It is predicted that by 2030 diabetes mellitus may afflict up to 79.4 million individuals in India, while China (42.3 million) and the United States (30.3 million) will also see significant increases in those affected by the disease. India currently faces an uncertain future in relation to the potential burden that diabetes may impose upon the country [10].
It was initially thought that the markers of inflammation is only increased in severe cases of COPD. However, recent studies have shown that circulatory inflammatory markers increases irrespective of lung function impairment [11,12]. There is a strong association between COPD and inflammatory markers such as CRP, fibrinogen, and Tumour Necrosis factor-α (TNF-α). Various studies have shown a rise in the number of inflammatory markers like TNF-α, CRP, lipopolysaccharide-binding protein, lipid products, and inflammatory cells in peripheral blood [13-16]. Pulmonary inflammatory biomarkers in induced sputum, bronchoalveolar lavage, endobronchial biopsy have also been studied to correlate the association between COPD and systemic manifestation [17]. Markers associated with neutrophilic inflammation and pro-inflammatory markers such as IL-6, IL-β, Interferon-α (IFN-α) etc., are found to be significantly elevated [18]. The increase in circulating inflammatory markers in COPD has been considered as a part of the “spill over” of the inflammatory mediators from the pulmonary compartment which is primarily responsible for systemic inflammation [19].
Therefore, it is suggested that systemic inflammation may probably be the common pathogenic mechanism responsible for genesis of COPD and its other comorbidities such as metabolic syndrome. The role of the spill over hypothesis has been further emphasised in recent studies by studying the relationship between the various inflammatory biomarkers and pulmonary tissue-derived proteins such as surfactant D-derived proteins from pneumocyte-II. A recent study by Kim DK et al., studied the candidate Single Nucleotide Polymorphisms (SNPs) of two pneumoproteins Clara Cell secretory protein (CC16) and Surfactant Protein D (SP-D) which appear to be strongly correlate with COPD. However, the roles of such biomarkers are yet to be fully established [20].
Therefore, this study was planned to further enrich the information on relationship between two common entities of COPD and T2DM with the help of inflammatory markers of hs-CRP and TLC.
Materials and Methods
This cross-sectional study was conducted on randomly collected sample of 100 patients of COPD attending outpatient department at Department of Respiratory Medicine, LN Medical College and Research Centre, Bhopal, Madhya Pradesh, India, from May 2019 - April 2020 after approval from from Institutional Ethical Committee (LNMC&RC/Dean/2019/Ethics/027 dated: 04/04/2019).
Inclusion criteria: All known/newly diagnosed stable cases of COPD, ≥40 year of age who consented to participate, with no exacerbations or change in treatment in last six weeks with or without T2DM were included in the study.
Exclusion criteria: All patients who did not consented to participate, very severe hospitalised patients with multiple co-morbidities with history or presence of tuberculosis/bronchiectasis, had history of inflammatory diseases, such as malignancy, arthritis, connective tissue disorders or inflammatory bowel disease, with cardiovascular disease ischemic heart disease or heart failure, or peripheral arterial disease or acute and chronic infection, patients on systemic (oral/injectable) corticosteroids, on NSAIDs, who has history of recent surgery or trauma, pregnant women and patients having serum CRP >10 mg/dL were excluded.
Sample size calculation: At 95% Confidence Interval (CI) and 5% α errors, sample size found to be 100.
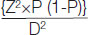
Z=Standard normal deviation, set at 1.96 to correspond to 95% confidence level p-value=prevalence of diseased=5% of α error
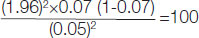
A total of COPD patients recruited after considering inclusion and exclusion criteria.
Only known patients of COPD or patients with complains of chronic cough was evaluated with chest x-ray and pulmonary function test (spirometry) after thorough history and physical examination. Hyperinflation in chest x-ray and postbronchodilator Forced Expiratory Volume (FEV1)/Forced Vital Capacity (FVC) ratio<0.7 (<70%) (for age >70 it is 0.65) on pulmonary function test by spirometer will be used for making diagnosis. Less than 12% or <200 mL pre to postbronchodilator reversibility in percent predicated value of FEV1 will be used for differentiating COPD with asthma. GOLD guidelines a grading system for COPD was used for assessing severity [21].
For assessing diabetes, the random blood sugar was taken by using fully automatic analyser and patients were classified as having the T2DM according to criteria adopted from ADA associated i.e., symptoms of diabetes plus random plasma glucose >200 mg/dL (11.1 mMol/L).
Venous blood samples were obtained to perform a quantitative hs-CRP estimation by immunoturbidimetry method and TLC by haematological autoanalyser was also done to know marker of systemic inflammation [22].
Statistical Analysis
Data was represented in frequency and percentage for non parametric parameters while parametric data will be represented as mean±standard deviations. Parametric data was compared using Student’s t-test while non parametric parameters were using chi-square test. Null hypothesis will be stand rejected if p-value is <0.05 with 95% of CI. Statistical analysis was performed using IBM SPSS version 26.0 of IBM Corporation, California, USA.
Results
In present study, 100 patients of COPD were studied for the demographic and clinical differences between non diabetic COPD group (non diabetic group) and diabetic COPD group (diabetic group). A comparative analysis was also done for inflammatory factors like TLC and hs-CRP levels between non diabetic and diabetic group of COPD patients.
Out of all 100 patients, 60 (60%) were non diabetic COPD patients; while rest 40 (40%) patients were in diabetic group being patients of both COPD and DM.
Demographic Data
The mean age for non diabetic patients were 55.88±8.03 years which was comparable to the mean age of diabetic group of patients with mean age of 54.5±7.62 years (p>0.05). Proportional distribution of patients in major age groups was also comparable between two groups (Chi-square=2.525; p-value=0.4708). Most common age group was 51-60 years of age with 27 (45%) patients in non diabetic age group while in the diabetic group most common age group was 41-50 years of age with 18 (45%) patients.
In the non diabetic group, 51 (85%) patients were males compared to 18 (45%) male patients in the diabetic group. Proportional distribution of males was significantly higher in the non diabetic group compared to the diabetic group (p<0.05) [Table/Fig-1]. Since the sample size was small, more males with COPD were found in non diabetic group by chance.
Comparing demographic features of non diabetic and diabetic COPD patients.
| Demographic charactersistics | Non diabetic (n=60) | Diabetic (n=40) | Statistical inference |
|---|
| Mean age in years (Mean±SD) | 55.88±8.03 | 54.5±7.62 | Unpaired student’s t-test; p>0.05 |
| Proportion of males (number, %) | 51 (85%) | 18 (45%) | Chi-square; p<0.0001 |
Most of the COPD patients were in GOLD-2 stage of COPD with 45 (45%) patients in the category, followed by 28 (28%) patients in GOLD-3 stage, 16 (16%) patients in GOLD-1 stage and 11 (11%) patients in GOLD-4 stage of COPD. A significantly higher proportions of patients were in GOLD-3 stages of non diabetic group compared to diabetic group (p<0.05) [Table/Fig-2]. Mean FEV1 of non diabetic group was significantly lower (p<0.05) than that of diabetic groups. As expected, patients diagnosed with COPD had FEV1 (% of predicted) and FVC (% of predicted) values lower than the subjects in control group (p<0.0001).
Showing distribution of patients according to severity of COPD between non diabetic and diabetic COPD patients.
| GOLD staging | Non diabetic (n=60) | Diabetic (n=40) | Total (n=100) |
|---|
| GOLD 1 | 4 (6.67%) | 12 (30%) | 16 (16%) |
| GOLD 2 | 24 (40%) | 21 (52.5%) | 45 (45%) |
| GOLD 3 | 23 (38.33%) | 5 (12.5%) | 28 (28%) |
| GOLD 4 | 9 (15%) | 2 (5%) | 11 (11%) |
| Total (n=100) | 60 (60%) | 40 (40%) | 100 (100%) |
Chi-square; df:16.90, 3; p-value=0.0007
Inflammatory Markers
High Sensitivity C-Reactive Protein (hs-CRP): The mean hs-CRP levels for diabetic group was significantly higher (p<0.05) than that of non diabetic group [Table/Fig-3].
Showing mean hs-CRP levels between non diabetic and diabetic COPD patients.
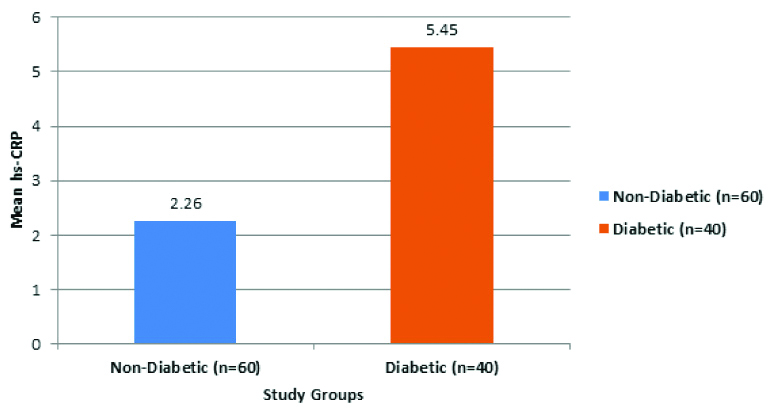
In the GOLD-1 stage of COPD of diabetic group had a mean hs-CRP levels of 4.66±0.55 mg/L, which was significantly higher (p<0.05) than 1.55±0.52 mg/L of non diabetic group. In the GOLD-2 stage of COPD of diabetic group had a mean hs-CRP levels of 5.62±0.91 mg/L, which was significantly higher (p<0.05) than 1.88±0.3 mg/L of non diabetic group. In the GOLD-3 stage of COPD of diabetic group had a mean hs-CRP levels of 6.14±1.7 mg/L, which was significantly higher (p<0.05) than mg/L of 2.26±0.36 non diabetic group. In the GOLD-4 stage of COPD of diabetic group had a mean hs-CRP levels of 6.55±0.35 mg/L, which was significantly higher (p<0.05) than 3.61±0.28 mg/L of non diabetic group [Table/Fig-4].
Showing mean hs-CRP levels between non diabetic and diabetic COPD patients according to GOLD staging.
The mean hs-CRP tend to had a positive correlation with increasing severity of GOLD severity from GOLD-1 to GOLD-4 in both non diabetic and diabetic groups.
Mean hs-CRP levels had a significant (p<0.05) positive correlation with TLC (r=0.7552) in the patients of non diabetic group; while mean hs-CRP levels had a significant (p<0.05) positive correlation with TLC (r=0.8973) in the patients of diabetic group [Table/Fig-5].
Showing correlation of hs-CRP with other demographic, clinical, metabolic and inflammatory parameters in diabetic patients.
| Parameter | Pearson R | R squared | p (two-tailed) |
|---|
| TLC | 0.8973 | 0.8052 | <0.0001 |
| Age | 0.4909 | 0.241 | 0.0013 |
| Pack per year | 0.1399 | 0.01957 | 0.3893 |
| BMI (kg/m2) | -0.1865 | 0.03477 | 0.2493 |
| WHR | -0.2788 | 0.07771 | 0.0857 |
| Fasting | 0.001458 | 0.000002125 | 0.9929 |
| Random | -0.0186 | 0.0003461 | 0.9093 |
| HbA1C (%) | 0.4423 | 0.1956 | 0.0043 |
| FEV1 | -0.593 | 0.3516 | <0.0001 |
| FEV1/FVC | 0.02664 | 0.0007099 | 0.8704 |
Discussion
In present 100 patients of COPD were studied where 60 (60%) were non diabetic patients and had COPD only; while rest 40 (40%) patients were in diabetic group being patients of both COPD and DM. To assess systemic inflammation authors used hs-CRP and TLC in present study.
Incidence of DM
In survey, the prevalence of T2DM in COPD patients was 21% which was far lower than the 40% of present study [23]. International Diabetes Federation and data from the National Registry of diabetes (Macedonia) shows that diabetes affects 2-37% of patients with COPD [24]. In a follow-up study pre-existing T2DM was identified in 16% patients on COPD diagnosis, later in 10 years this data become 34% and comparable to present study [1]. A study from Karnataka reported a prevalence of 25.63% of DM in COPD patients [25]. While a study from Hyderabad reported 32.28% prevalence of diabetics’ in COPD patients [26]. The incidence of DM was 10.3% in a population of grade 2/3 COPD patients in a rehabilitation centre according to a study conducted at Italy, 12.6-14.5% in an all-stage COPD population according to Celli BR et al., while 12.2% with an increased risk in active smokers according to Mannino DM et al., [27-29]. However the high prevalence of DM in the current study could be due to the fact that most of the patients age was more than 50 years with history of smoking or exposure to biomass fuel for more than 10 years.
Demographic Data
A study from central Karnataka India had comparable mean age of 58±9.6 years in COPD patients [25]. Most of the patients were in higher age group of more than 50 years of age in present study, while most of in age group more than 70 years of age in another study [1]. Mean age in COPD group was reported to be 64.7 years by Silva DR et al., which were higher than present study [30]. Higher age groups may be due to different selection criterion or socio-demographic differences in populations e.g. smoking habits include bidi, hukka, chilam etc., in our region while cigarette etc., in western countries.
Out of all patients of COPD, 69 (69%) were males and 31 (31%) were females making male to female ratio of about 7:3. Significantly more males were in non diabetic group 51 (85%) than diabetic group which had 18 (45%) males (p>0.05). In Brazil there were 61.67% males and 38.33% female in COPD group and 61% males in diabetic group while 72% males were in the non diabetic [30]. Naseem S and Baneen U reported a comparable male to female ratio while in present study groups COPD only had higher ratio of males [31]. The probable cause could be more male smokers in Indian population while comparable ratio in COPD patients with DM may be due to increase inflammatory response in the group as suggested by higher hs-CRP and TLC levels. Although comparable results to present study was seen with 66% males and females 34% females in a study conducted at Macedonia where men with severe and very severe COPD had insignificant higher average value of blood glucose than women (5.68±0.9 vs. 5.54±1.0 and 8.0±3.6 vs. 6.03±1.3, consequently) [23]. In another study from Karnataka, found 76.05% males and 23.95% females of COPD in the study [25]. Since the sample size was small, so may be more females with COPD found into the diabetic group by chance.
Severity of COPD
The GOLD stage-2 patients were commonest in both non diabetic and diabetic groups, 24 (40%) and 21 (52.5%) patients respectively. There were 89% mild category patients in diabetic group while same were 91% in the non diabetic group in a study by Ho T-W et al. which were comparable to present study [1]. A study conducted in south India reported no GOLD stage I (due to exclusion criteria), GOLD stage II had 22.04%, GOLD stage III had 33.07 and 44.88% were in GOLD stage IV, which were in inverse ratio than present study [26]. According to the GOLD criteria, there were 2 (3.3%), 20 (33.3%), 21 (35.0%), and 17 (28.3%) patients with stages I, II, III, and IV disease, respectively by a study at Brazil by Silva DR et al., [30]. In a study conducted on COPD patients at Serbia, Belgrade by Vujic T et al., found that prevalence of 33.3%, 48.8%, 31.6%, and 23.1% in GOLD stages I, II, III, and IV, respectively [32]. That difference in GOLD staging distribution from above mention studies could be attributed to different study structure than our present study.
Inflammatory Markers
High Sensitivity C-Reactive Protein (hs-CRP): The mean hs-CRP levels for diabetic group was 5.45±1.07 mg/L, which was significantly higher (p<0.05) than 2.26±0.69 mg/L that of non diabetic group. Naseem S and Baneen U compared mean levels of hs-CRP in COPD and patients of COPD with metabolic syndrome, the COPD patients had mean hs-CRP of 3.88±0.72 mg/L which was significantly lower than later group of 5.06±0.59 mg/L. Those results were comparable to present study findings [31]. In a study done at Mysore India by Ramesh S et al., 7 (9%) patients had low hs-CRP levels, 15 (20%) had intermediate and 55 (71%) had high hs-CRP levels [33]. Pradhan AD et al., observed that inflammation increases the risk of developing T2DM. They studied 27,628 females to assess the impact of inflammatory biomarkers on the new onset T2DM. They found that the highest quartile of CRP gave a relative risk of 4.4 (95% CI 1.5-12.0) [34]. While high levels of hs-CRP (>3 mg/L) were associated with a significantly increased risk of COPD incident {Hazard Ratio (HR), 1.7; 95% CI, 1.16-2.49} compared with persons with low levels (<1 mg/L) [35].
Study in Brazil by Silva DR et al., found significant positive correlations between BMI and CRP in COPD patients (r=0.3, p=0.045), Similar to present study negative correlation was found between hs-CRP and FEV1 (% of predicted). In COPD, no correlation was observed between FEV1 (% of predicted) and CRP (r=-0.094 and p=0.473 for COPD group; r=-0.20 and p=0.323 for control group) [30]. The GOLD staging of severity showed significant increase with each increasing severity scale thus significant negative relation to FEV1 similar to present study [36].
Mean hs-CRP levels had a significant positive correlation with TLC, Age and HbA1c, while significant negative correlation with FEV1, in the patients of diabetic group. Study conducted at four US communities by Yeh HC et al., reported that lower vital capacity is an independent predictor of incident type 2 diabetes. Pulmonary factors related to vital capacity deserve attention as possible risk factors for insulin resistance and diabetes [37]. Although it is not shown in present study results but it is proven that increase BMI is associated with decreased pulmonary function as shown by Lam K-BH et al., and Leone N et al., [38,39]. Contrast to present study, Ramesh S et al., observed that mean of Fasting Blood Sugar (FBS), Post Prandial Blood Sugar (PPBS) and HbA1c correlated positively with hs-CRP [33].
Patients with COPD are at risk of developing diabetes. COPD is also found to be a common comorbidity of diabetes. The correlation implies that components of the metabolic syndrome particularly hyperglycaemia can give rise to pulmonary function impairment in COPD and vice versa [31].
Limitation(s)
The sample size of the study was very small. So the result of the study cannot be generalised on the whole population. A study with large sample size is recommended.
Conclusion(s)
The prevalence of T2DM among COPD patients was found to be 40%. TLC and hs-CRP as inflammatory markers was well correlated with each other. The hs-CRP was significantly higher in the diabetic group. It was concluded that the inflammatory process is a definite pathophysiological factor that has a significant link between COPD and T2DM and can be evaluated using a marker like hs-CRP level.