Introduction
Catheter-Related Blood Stream Infections (CRBSI) are an important complication of both non tunnelled and tunnelled haemodialysis catheters, but are often poorly reported for tunnelled haemodialysis-catheters.
Aim
To assess the rate, aetiology, and outcomes of CRBSI in patients using a tunnelled catheter at 12-month and 18-month audits at the newly-opened haemodialysis unit having care bundle as a part of routine catheter care.
Materials and Methods
A retrospective cross-sectional study involving two audits of CRBSI risk (12-month and 18-month audit) was conducted by the dialysis unit doctors and nursing staff at Medanta Super-Specialty Private Hospital, Indore, Madhya Pradesh, India. Centres for Disease Control (CDC) and prevention core intervention/care bundle for Blood Stream Infections (BSI) reduction were incorporated as a part of routine catheter care. The 12-month (May 2018 to April 2019) and 18-month (May 2018 to November 2019) internal clinical audit were evaluated to assess the impact of care bundle on incidence of CRBSIs. Kidney-Disease-Outcome Quality-Initiative (KDOQI)-2006-criteria was used to define CRBSI. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 19.0 software (IBM Corporation, New York, United States). Descriptive and dispersion statistical analysis was done for studied variables.
Results
Total patients in 12-months audit with tunnelled haemodialysis catheter were 14 (7 male and 7 female) with median age 64 years and in 18-months audit patient with tunnelled haemodialysis catheter were 18 (11 male and 7 female) with median age 67.5 years. CRBSI incidence was 2.58 per 1000 catheter days at the end of 12-month, with 132 (71.25-202.25) days of median catheter use. Over the 18-month, the incidence of CRBSI dropped to 1.99 per 1000 catheter days. Median period of catheter use increased to 149.5 (83.5-294.5) days. The primary organisms isolated were predominantly gram negative bacterias.
Conclusion
Tunnelled catheters may be a reasonable alternative vascular access for haemodialysis in patients with arteriovenous fistula failure as implementation and maintenance of multidisciplinary care bundle reduces CRBSI rate in such patients.
Introduction
The use of tunnelled Central Venous Catheters (CVC) (or permanent catheters) as haemodialysis access has increased considerably among Indian patients with end-stage renal disease despite strong recommendations for Arterio-Venous Fistula (AVF) [1]. Their prolonged dependence is often complicated by Catheter-Related Blood Stream Infections (CRBSIs), with incidence rates ranged from 0.19-5.5 per 1000 catheter days [2-6].
The CRBSIs may lead to prolonged hospitalisation, increases in costs, morbidity and mortality rates in patients undergoing haemodialysis through catheters [7,8]. In 2003, a five year prospective nested case-control study from Argentina found an additional cost of $ 4888.42 and an increase in hospital stay of 11.9 days for each episode [9].
Although, there is no consensus on the optimal approach to reduce the incidence of CRBSIs, several studies indicate that implementing care bundles and stringent surveillance can decrease the incidence of CRBSI by up to 80%, reaching a rate of zero in some cases [10-13]. However, audits of dialysis unit that has incorporated a multidisciplinary care approach to vascular access and infection management are underestimated and infrequently performed in India [14]. Also, continuous auditing play a vital role in the improvement of adherence to multidisciplinary care approach [15].
For this reason, this audit was conducted in one of the newly opened Indian haemodialysis unit to improve the adherence to multidisciplinary care approach in haemodialysis unit staff and to reduce the incidence of CRBSIs in haemodialysis patients.
Materials and Methods
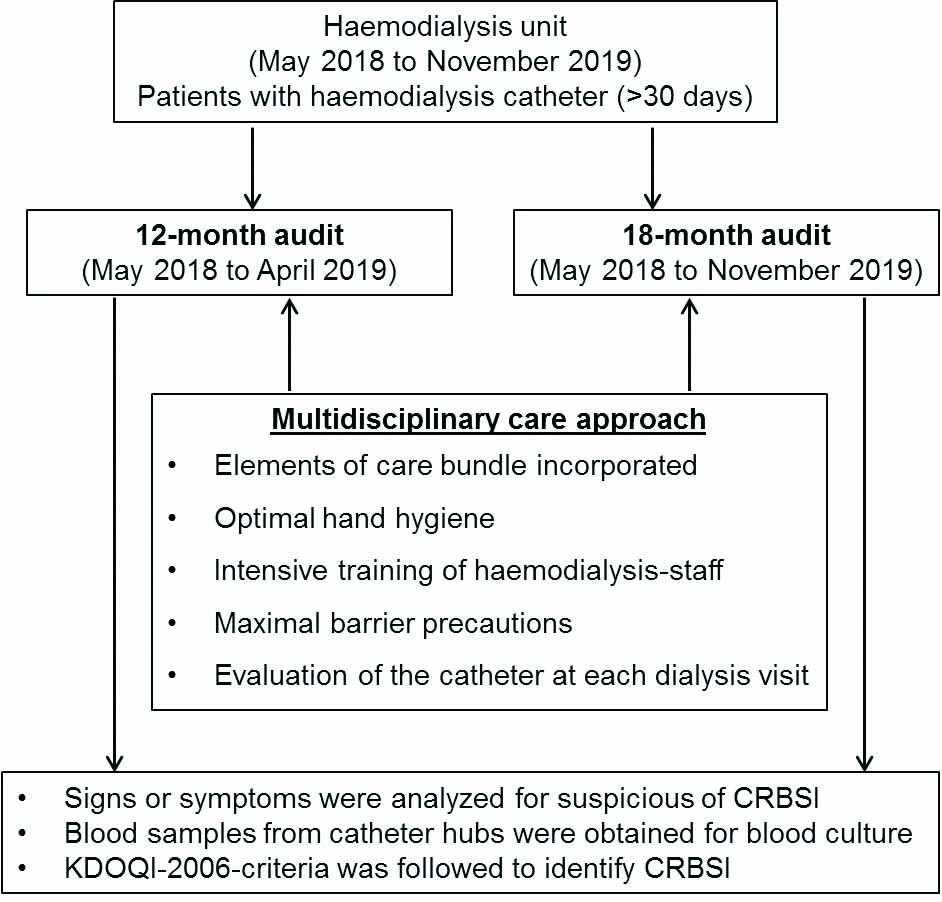
This retrospective cross-sectional study involving two audits (1st May 2018 to 30th April 2019 and 1st May 2018 to 30th November 2019) was conducted at the newly-opened Haemodialysis Unit of a Medanta Super-Specialty Private Hospital, Indore, Madhya Pradesh, India. The unit has incorporated a multidisciplinary care approach to vascular access and infection management. Patients who were undergoing haemodialysis and had tunnelled haemodialysis catheter for more than 30 days were selected for audit. The audit was conducted by the dialysis unit doctors for the incidence, and aetiology of CRBSI on two different occasions as presented in [Table/Fig-1].
Aetiology of CRBSI in patients with tunnelled haemodialysis catheter during two audits.
The audit was performed in accordance to Helsinki declaration for the ethical guidelines of humans in medical and health experimentation. The necessity of ethical approval was waived as the audit involved collection of existing data from patient records. The records were anonymised before the data analysis.
Audits
During the 12-month audit, the case-notes of all patients fulfilling the eligibility criteria and had haemodialysis between May 2018 and April 2019 were reviewed in May 2019 and baseline figures were recorded for the incidence of CRBSIs, duration of catheter use and aetiology of CRBSI. Re-audit was done between May 2018 and November 2019 which included patients undergoing haemodialysis. In this 18-month audit, the same parameters were recorded by the dialysis unit doctors. The parameters were assessed as per the routine protocol of the institute.
Multidisciplinary Care Approach
Centres for Disease Control (CDC) and prevention core interventions for Blood Stream Infections (BSI) reduction were incorporated as a part of routine catheter care in the newly-opened haemodialysis unit of the hospital [16]. These include surveillance and feedback using National Healthcare Safety Network (NHSN), hand hygiene observations, catheter/vascular access care observations, staff education and competency, patient education/engagement, catheter reduction, chlorhexidine for skin antisepsis, catheter hub disinfection and antimicrobial ointment [16]. Full-time Infection Control Nurses (ICNs) were trained by infection control officer to monitor the adherence of each element of this core intervention or bundle care. The healthcare personnel were educated regarding the importance of each element of bundle care and training was given as per the need of the centre. Training of implementation of catheter care bundle (both insertion and maintenance bundle) was conducted in batches, both for clinicians and paramedical staff. The 12-month and the 18-month internal clinical audits were evaluated to assess the impact of care bundle on incidence of CRBSIs.
Catheter-Related Blood Stream Infections (CRBSI)
The healthcare professionals screened patients for vascular access related infection as part of standard procedure during each haemodialysis session. The standard institutional protocol was implemented when the patient displayed signs or symptoms suspicious of CRBSI before or during the haemodialysis session, which included fever (>38.0°C before dialysis and >37.7°C during dialysis), chills, rigors, hypotension, and new unexplained malaise, with concurrent exclusion of catheter unrelated infectious foci. In such patients, the haemodialysis was stopped for as long as necessary to obtain blood samples from catheter hubs (typically ≤1 minute). Haemodialysis was not stopped to obtain peripheral vein or haemodialysis circuit blood cultures. Kidney Disease Outcomes Quality Initiative (KDOQI) 2006 criteria were used to define CRBSI [17]. A bloodstream infection was defined as a positive culture from the catheter with/without a positive peripheral venipuncture sample along with symptoms and signs of a blood stream infection. KDOQI recommendations for management of CRBSI’s were followed.
For all patients, clinical and demographical data, including age, sex, history of diabetes, and hypertension were collected.
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 19.0 software (IBM Corporation, New York, United States). Descriptive and dispersion statistical analysis was done for studied variables. Absolute frequency (raw counts) was used to denote CRBSI episodes in two audits. Relative frequency (% of the total number of observation) was calculated for number of male and female patients, cases of diabetes and hypertension, and for number of isolated microorganisms. Dispersion statistics {median (Interquartile range)} was calculated for age of the patients and period of permanent tunnelled catheter use. Incidence of CRBSI was presented as CRBSI rate per 1000 catheter days.
Results
The characteristics of the patients enrolled during 12-month and 18-month audits are presented in [Table/Fig-2]. Over the first 12 month, out of 26 patients on regular maintenance haemodialysis, 14 (53.85%, 7 males and 7 females) had AVF-failure or poor veins and were on tunnelled haemodialysis catheter. The median age was 64 (IQR, 49-70.5) years, and diabetes (78.57%) and hypertension (64.29%) were the most prevalent comorbid conditions. The median period of catheter-use was 132 (IQR, 71.25-202.25) days. Five episodes of CRBSI were identified in four patients; one patient being immunosuppressed had a recurrent infection and lost the catheter to candida infection. The incidence of CRBSI was 2.58 per 1000 catheter days during the first audit [Table/Fig-3].
Characteristics of the patients with permanent catheter.
| Characteristics | 12-month audit | 18-month audit |
|---|
| Total patients | 14 | 18 |
| Male, n (%) | 7 (50%) | 11 (61.11%) |
| Female, n (%) | 7 (50%) | 7 (38.89%) |
| Median age (IQR) (years) | 64 (49-70.5) | 67.5 (49-69) |
| Diabetes mellitus, n (%) | 10 (71.42%) | 14 (77.78%) |
| Hypertension, n (%) | 9 (64.29%) | 11 (61.11%) |
CRSBI rate in first data collection period and in second data collection period.
| Variables | 12-month audit (n=14) | 18-month audit (n=18) |
|---|
| CRBSI episodes, numbers | 5 | 8 |
| CRBSIs rate per 1000 catheter days | 2.58 | 1.99 |
| Period of permanent tunnelled catheter use in days, median (IQR) | 132 (71.25-202.25) | 149.5 (83.5-294.5) |
CRBSI: Catheter-related bloodstream infection
A total of 29 patients undergoing haemodialysis were included in the re-audit period. Of these 29 patients, 18 (62.07%; 11 males and 7 females) were on the tunnelled haemodialysis catheter; 14 continued from the previous audit and four patients fulfilling the eligibility criteria were newly included during the next audit. Two episodes of CRBSI were reported during next six months while prevalence estimate for patients with diabetes and hypertension were 77.78% and 61.11% respectively. The median period of catheter use increased from 132 (IQR, 71.25-202.25) at the first audit to 149.5 (IQR, 83.5-294.5) days at the second audit. The incidence of CRBSI reduced to 1.99 per 1000 catheter days at the second audit [Table/Fig-3].
In the second audit, the primary microbes isolated were gram negative organisms as shown in [Table/Fig-4]. The common microorganisms isolated from CRBSI infection cases were Escherichia coli (14.29%), Candida tropicalis (14.29%), Burkholderia cepecia (28.57%), Entobacter cloacae (14.29%), Staphylococcus aureus (14.29%) and Staphylococcus epidermidis (14.29%). Total 57.14% (4/7) were identified as gram negative organisms, obtained from positive blood cultures in this audit along with 28.57% of gram positive organisms (2/7).
Isolated microorganisms from CRBSI cases.
| Microorganisms | 12-month audit (n=5) | 18-month audit (n=7) |
|---|
| Escherichia coli, n (%) | 1 (20%) | 1 (14.29%) |
| Candida tropicalis, n (%) | 1 (20%) | 1 (14.29%) |
| Enterobacter cloacae, n (%) | 1 (20%) | 1 (14.29%) |
| Burkholderia cepecia, n (%) | 1 (20%) | 2 (28.57%) |
| Staphylococcus aureus, n (%) | 1 (20%) | 1 (14.29%) |
| Staphylococcus epidermidis, n (%) | | 1 (14.29%) |
| Total | 5 (100%) | 7 (100%) |
Discussion
The medical audit is a process of reviewing the delivery of health care to identify deficiencies so that it may be remedied. This audit demonstrated that introduction and maintenance of multidisciplinary care bundle appears to increase median duration of catheter use and reduce CRBSI. There was a gradual increase in duration of catheter use and decrease in CRBSI as shown by the first and second audits at the newly opened haemodialysis unit of the study hospital.
National and international guidelines along with national policy initiatives recommend the use of AVF whenever possible, as the risk of blood stream infection is highest in patients with CVCs [18-23]. Previous studies reported approximately 2.5-10 times higher incidence of infection with permanent catheter than AVF [2,24]. However, poor vein and AVF failure were the major reasons to tunnel catheter use among haemodialysis patients in the study unit. The hub of the catheter is a major source of colonisation leading to CRBSI [25]. Further, the comparison of the peripheral blood culture with blood cultures obtained simultaneously from the arterial or venous CVC are frequently impractical in patients on dialysis; blood cultures obtained from the dialysis circuit or catheter lumen are as sensitive and specific, and reasonable alternative [26]. Thus, blood cultures were obtained from the catheter hub in this audit only to salvage catheters and preserve vascular access.
Studies have reported infection rates of about 0.5-5.5 events per 1000 catheter days for tunnelled cuffed catheters [24,27-30]. In 2017, Lok CE classified facility performance based on CRBSIs rate and kept facility with CRBSI rate of 2.1-3.0 episodes per 1000 catheter days under good category [31]. However, the facility with CRBSI rate of <1 and 1.0-2.0 episodes per 1000 catheter days were categorised as excellent and very good [31]. The present audits showed a decrease in CRBSI rate from 2.58 events/1000 catheter days at the first 12-month to 1.99 events/1000 catheter days at the second 18-month, achieving a 22.87% reduction rate. Previous studies have also reported lower CRBSI rates after implementation of control bundles [32-34]. Hymes JL et al., reported a significant reduction in CRBSI to 0.67 per 100 patient months with the use of antimicrobial barrier caps [35]. In 2020, the findings from the Standardising Care to Improve Outcomes in Paediatric End Stage Renal Disease (SCOPE) collaborative showed a significant decrease in adjusted CRBSI rate from 3.3/100 patient months to 0.8/100 patient months after 48 months of care bundle implementation (p<0.001) [36]. The reduction in infection rate is surely attributable to increased skill and awareness of healthcare professionals as well as increased compliance with the surveillance and multidisciplinary care bundle elements. The encouraging findings emphasise the need for sustained quality improvement initiatives.
The risk of CRBSI increases with the duration of CVC dependence. A study of 472 haemodialysis patients receiving a first ever tunnelled dialysis catheter found CRBSI in 35%, 54%, and 79% of patients at three, six, and 12 months, respectively [37]. In contrast, the present audit showed 35.71% (5/14) CRBSI in positive culture at 12-month audit and 38.89% (7/18) at 18-month audit.
An increase in median period of catheter use from 132 days during initial 12-month to 149.5 days, over 18-month was found in this audit. However, a previous study involving the use of tunnelled catheter has reported a median period of seven months [1]. Thus, tunnelled catheters may be an alternative for patients with poor veins and having limited options for AV-Fistula/graft.
Although, previous studies from various countries have reported gram negative bacterial growth in 15% to 26% of positive cultures [24,38-42], Gram negative organisms were the predominant microbes (57.14%) from positive blood cultures in our audit, with only a smaller proportion of CRBSIs attributable to gram positive organisms (28.57%). The finding is consistent with few Indian studies who reported gram negative pathogens as the major causative agents for CRBSIs [43,44]. The proportion of gram negative CRBSI was much higher than that reported in western hospitals [43]. The higher prevalence of gram negative pathogens is mainly facilitated by poor hand hygiene, water contamination and inadequate disinfection or sterilisation of instruments or surfaces [44].
Limitation(s)
There are some limitations of this clinical audit. In most instances, only one sample per patient was obtained. The patients who had a recent catheter placement were not excluded. This might not have given enough time for the catheter to become colonised, as endoluminal colonisation of catheters may increase with time. Finally, in dialysis patients the outer surface of the extravascular segment of the catheter, rather than the endoluminal surface, may have a higher microbiological yield.
Conclusion(s)
In conclusion, tunnelled catheters may be used for vascular access in haemodialysis patients with arteriovenous fistula failure. Introducing and maintaining a multidisciplinary care bundle can lead to reduction in CRBSI.
CRBSI: Catheter-related bloodstream infection
[1]. Sampathkumar K, Ramakrishnan M, Sah A, Sooraj Y, Mahaldhar A, Ajeshkumar R, Tunneled central venous catheters: Experience from a single centerIndian J Nephrol 2011 21(2):10710.4103/0971-4065.8213321769173 [Google Scholar] [CrossRef] [PubMed]
[2]. Taylor G, Gravel D, Johnston L, Embil J, Holton D, Paton S, Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patientsAm J Infect Control 2004 32(3):155-60.10.1016/j.ajic.2003.05.00715153927 [Google Scholar] [CrossRef] [PubMed]
[3]. Kher V, Tunneled central venous catheters for dialysis–A necessary evil?Indian J Nephrol 2011 21(4):22110.4103/0971-4065.8548022022079 [Google Scholar] [CrossRef] [PubMed]
[4]. Thompson S, Wiebe N, Klarenbach S, Pelletier R, Hemmelgarn BR, Gill JS, Catheter-related blood stream infections in hemodialysis patients: A prospective cohort studyBMC Nephrology 2017 18(1):01-08.10.1186/s12882-017-0773-529221439 [Google Scholar] [CrossRef] [PubMed]
[5]. Shah S, Singhal T, Naik R, Thakkar P, Incidence and etiology of hemodialysis catheter related blood stream infections at a tertiary care hospital in Mumbai: A 5 year reviewIndian J Nephrol 2020 30(2):13210.4103/ijn.IJN_127_1932269441 [Google Scholar] [CrossRef] [PubMed]
[6]. Heidempergher M, Sabiu G, Orani MA, Tripepi G, Gallieni M, Targeting COVID-19 prevention in hemodialysis facilities is associated with a drastic reduction in central venous catheter-related infectionsJ Nephrol 2021 34(2):345-53.10.1007/s40620-020-00900-333369726 [Google Scholar] [CrossRef] [PubMed]
[7]. Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, Kiaii M, Hemodialysis tunneled catheter-related infectionsCan J Kidney Health Dis 2016 3:205435811666912910.1177/205435811666912928270921 [Google Scholar] [CrossRef] [PubMed]
[8]. Martin K, Lorenzo YSP, Leung PYM, Chung S, O’flaherty E, Barker N, Clinical outcomes and risk factors for tunneled hemodialysis catheter-related bloodstream infectionsOpen forum infectious diseases 2020 7(6)USOxford University Press:ofaa11710.1093/ofid/ofaa11732550235 [Google Scholar] [CrossRef] [PubMed]
[9]. Rosenthal VD, Guzman S, Migone O, Crnich CJ, The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina: A prospective, matched analysisAm J Infect Control 2003 31(8):475-80.10.1016/j.ajic.2003.03.00214647110 [Google Scholar] [CrossRef] [PubMed]
[10]. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MNI, Lamb-Jenkins J, Athan E, Effectiveness of a care bundle to reduce central line-associated bloodstream infectionsMed J Aust 2015 202(5):247-49.10.5694/mja14.0164425758694 [Google Scholar] [CrossRef] [PubMed]
[11]. Exline MC, Ali NA, Zikri N, Mangino JE, Torrence K, Vermillion B, Beyond the bundle-journey of a tertiary care medical intensive care unit to zerocentral line-associated bloodstream infectionsCritical Care 2013 17(2):01-13.10.1186/cc1255123497591 [Google Scholar] [CrossRef] [PubMed]
[12]. Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, An intervention to decrease catheter-related bloodstream infections in the ICUN Engl J Med 2006 355(26):2725-32.10.1056/NEJMoa06111517192537 [Google Scholar] [CrossRef] [PubMed]
[13]. Padilla Fortunatti CF, Impact of two bundles on central catheter-related bloodstream infection in critically ill patientsRevista Latino-Americana De Enfermagem 2017 25:e295110.1590/1518-8345.2190.295129211195 [Google Scholar] [CrossRef] [PubMed]
[14]. Bansal D, Kher V, Gupta KL, Banerjee D, Jha V, Haemodialysis vascular access: Current practices amongst Indian nephrologistsThe J Vasc Access 2018 19(2):172-76.10.5301/jva.500081729192721 [Google Scholar] [CrossRef] [PubMed]
[15]. Sastry AS, Deepashree R, Bhat P, Impact of a hand hygiene audit on hand hygiene compliance in a tertiary care public sector teaching hospital in South IndiaAm J Infect Control 2017 45(5):498-501.10.1016/j.ajic.2016.12.01328131421 [Google Scholar] [CrossRef] [PubMed]
[16]. Control CfD, Prevention. CDC approach to BSI prevention in dialysis facilities (ie, the core interventions for dialysis bloodstream infection [BSI] prevention). 2014 [Google Scholar]
[17]. Clinical practice guidelines for vascular accessAm J Kidney Dis 2006 48(Suppl 1):S176-247.10.1053/j.ajkd.2006.04.02916813989 [Google Scholar] [CrossRef] [PubMed]
[18]. Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Associations between hemodialysis access type and clinical outcomes: A systematic reviewJ Am Soc Nephrol 2013 24(3):465-73.10.1681/ASN.201207064323431075 [Google Scholar] [CrossRef] [PubMed]
[19]. Vassalotti JA, Jennings WC, Beathard GA, Neumann M, Caponi S, Fox CH, Fistula first breakthrough initiative: targeting catheter last in fistula firstSemin Dial 2012 25(3):303-10.10.1111/j.1525-139X.2012.01069.x22487024 [Google Scholar] [CrossRef] [PubMed]
[20]. Navuluri R, Regalado S, The KDOQI 2006 Vascular access update and fistula first program synopsisSemin Intervent Radiol 2009 26(2):122-24.10.1055/s-0029-122245521326502 [Google Scholar] [CrossRef] [PubMed]
[21]. Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Hemodialysis clinical practice guidelines for the Canadian Society of NephrologyJ Am Soc Nephrol 2006 17(3 Suppl 1):S01-27. [Google Scholar]
[22]. Napalkov P, Felici DM, Chu LK, Jacobs JR, Begelman SM, Incidence of catheter-related complications in patients with central venous or hemodialysis catheters: A health care claims database analysisBMC Cardiovasc Disord 2013 13:8610.1186/1471-2261-13-8624131509 [Google Scholar] [CrossRef] [PubMed]
[23]. Lacson E, Wang W, Lazarus JM, Hakim RM, Change in vascular access and hospitalisation risk in long-term hemodialysis patientsClin J Am Soc Nephrol 2010 5(11):1996-2003.10.2215/CJN.0896120920884778 [Google Scholar] [CrossRef] [PubMed]
[24]. Fysaraki M, Samonis G, Valachis A, Daphnis E, Karageorgopoulos DE, Falagas ME, Incidence, clinical, microbiological features and outcome of bloodstream infections in patients undergoing hemodialysisInt J Med Sci 2013 10(12):1632-38.10.7150/ijms.671024151435 [Google Scholar] [CrossRef] [PubMed]
[25]. Soi V, Moore CL, Kumbar L, Yee J, Prevention of catheter-related bloodstream infections in patients on hemodialysis: Challenges and management strategiesInt J Nephrol Renovasc Dis 2016 9:95-103.10.2147/IJNRD.S7682627143948 [Google Scholar] [CrossRef] [PubMed]
[26]. Quittnat Pelletier F, Joarder M, Poutanen SM, Lok CE, Evaluating approaches for the diagnosis of hemodialysis catheter-related bloodstream infectionsClin J Am Soc Nephrol 2016 11(5):847-54.10.2215/CJN.0911081527037271 [Google Scholar] [CrossRef] [PubMed]
[27]. Betjes MG, Prevention of catheter-related bloodstream infection in patients on hemodialysisNat Rev Nephrol 2011 7(5):257-65.10.1038/nrneph.2011.2821423251 [Google Scholar] [CrossRef] [PubMed]
[28]. Katneni R, Hedayati SS, Central venous catheter-related bacteremia in chronic hemodialysis patients: Epidemiology and evidence-based managementNat Clin Pract Nephrol 2007 3(5):256-66.10.1038/ncpneph044717457359 [Google Scholar] [CrossRef] [PubMed]
[29]. Rabindranath KS, Bansal T, Adams J, Das R, Shail R, MacLeod AM, Systematic review of antimicrobials for the prevention of haemodialysis catheter-related infectionsNephrol Dial Transplant 2009 24(12):3763-74.10.1093/ndt/gfp32719592599 [Google Scholar] [CrossRef] [PubMed]
[30]. Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti-Monros A, Catheter-Related Blood Stream Infections (CRBSI): A European viewNephrol Dial Transplant 2010 25(6):1753-56.10.1093/ndt/gfq20520466662 [Google Scholar] [CrossRef] [PubMed]
[31]. Lok CE, Management of a patient with catheter-related bloodstream infectionClin J Am Soc Nephrol 2017 12(11):1873-77.10.2215/CJN.0121021728679561 [Google Scholar] [CrossRef] [PubMed]
[32]. Yi SH, Kallen AJ, Hess S, Bren VR, Lincoln ME, Downham G, Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effortInfect Control Hosp Epidemiol 2016 37(7):863-66.10.1017/ice.2016.2226868605 [Google Scholar] [CrossRef] [PubMed]
[33]. Menegueti MG, Ardison KMM, Bellissimo-Rodrigues F, Gaspar GG, Martins-Filho OA, Puga ML, The impact of implementation of bundle to reduce catheter-related bloodstream infection ratesJ Clin Med Res 2015 7(11):85710.14740/jocmr2314w26491498 [Google Scholar] [CrossRef] [PubMed]
[34]. Lobo RD, Levin AS, Gomes LMB, Cursino R, Park M, Figueiredo VB, Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical intensive care unit in BrazilAm J Infect Control 2005 33(2):83-87.10.1016/j.ajic.2004.05.00315761407 [Google Scholar] [CrossRef] [PubMed]
[35]. Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D, Dialysis catheter-related bloodstream infections: A cluster-randomized trial of the clearguard HD antimicrobial barrier capAm J Kidney Dis 2017 69(2):220-27.10.1053/j.ajkd.2016.09.01427839894 [Google Scholar] [CrossRef] [PubMed]
[36]. Marsenic O, Rodean J, Richardson T, Swartz S, Claes D, Day JC, Tunneled hemodialysis catheter care practices and blood stream infection rate in children: Results from the SCOPE collaborativePediatr Nephrol 2020 35(1):135-43.10.1007/s00467-019-04384-731654224 [Google Scholar] [CrossRef] [PubMed]
[37]. Shingarev R, Barker-Finkel J, Allon M, Natural history of tunneled dialysis catheters placed for hemodialysis initiationJ Vasc Interv Radiol 2013 24(9):1289-94.10.1016/j.jvir.2013.05.03423871694 [Google Scholar] [CrossRef] [PubMed]
[38]. Murea M, James KM, Russell GB, Byrum GV 3rd, Yates JE, Tuttle NS, Risk of catheter-related bloodstream infection in elderly patients on hemodialysisClin J Am Soc Nephrol 2014 9(4):764-70.10.2215/CJN.0771071324651074 [Google Scholar] [CrossRef] [PubMed]
[39]. Nguyen DB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, National Healthcare Safety Network (NHSN) Dialysis Event Surveillance Report for 2014Clin J Am Soc Nephrol 2017 12(7):1139-46.10.2215/CJN.1141111628663227 [Google Scholar] [CrossRef] [PubMed]
[40]. Patel PR, Shugart A, Mbaeyi C, Goding Sauer A, Melville A, Nguyen DB, Dialysis Event Surveillance Report: National Healthcare Safety Network data summary, January 2007 through April 2011Am J Infect Control 2016 44(8):944-47.10.1016/j.ajic.2016.02.00927040568 [Google Scholar] [CrossRef] [PubMed]
[41]. Fram D, Okuno MF, Taminato M, Ponzio V, Manfredi SR, Grothe C, Risk factors for bloodstream infection in patients at a Brazilian hemodialysis center: A case-control studyBMC Infect Dis 2015 15:15810.1186/s12879-015-0907-y25879516 [Google Scholar] [CrossRef] [PubMed]
[42]. Loo LW, Liew YX, Choong HL, Tan AL, Chlebicki P, Microbiology and audit of vascular access-associated bloodstream infections in multi-ethnic Asian hemodialysis patients in a tertiary hospitalInfect Dis (Lond) 2015 47(4):225-30.10.3109/00365548.2014.98619325664373 [Google Scholar] [CrossRef] [PubMed]
[43]. Gopalakrishnan R, Sureshkumar D, Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programmeJ Assoc Physicians India 2010 58(Suppl):25-31. [Google Scholar]
[44]. Pattanashetti N, Ramachandran R, Kohli HS, Gupta KL, Hemodialysis tunneled catheter-related infection in a tertiary care center: A changing trendSaudi J Kidney Dis Transpl 2019 30(5):1187-89.10.4103/1319-2442.27028031696863 [Google Scholar] [CrossRef] [PubMed]