Quality maintenance is a “societal responsibility” which should be included as a component of continuous quality initiative. It is defined as the consistent and reliable performance of services or products in conformance to the specified standards [1]. Several approaches to perform QC of BC have been promulgated by the American Association of Blood Bank (AABB), the Council of Europe (CE), and Director General of Health Services (DGHS) India. These are among the few that provide benchmarks for meeting the quality of BC [2]. Of all the blood components, platelets are considered as “perishable” because of a shorter life-span. The platelet morphology and function maybe affected during the processing or storage leading to the development of Platelet Storage Lesion (PSL) which can be less effective when compared to fresh PC. The platelets in the individual units must be viable and adequate in number to be effectively transfused [3]. Hence, this study was performed on comparative analysis of PC prepared by PRP, BC and apheresis methods to ascertain the various in-vitro quality and various other biochemical parameters of platelets so produced to establish the optimum quality standards in order to provide the maximum therapeutic benefit to patients.
The basic objectives of the study was to prepare PCs by 3 different methodologies consisting of PRP, BC and apheresis methods and also to undertake comparative evaluation of the quality parameters of 3 categories of PCs obtained respectively.
Materials and Methods
This study was laboratory based observational study which was conducted at RL Jalappa Hospital blood bank, Kolar, Karnataka from December 2018 to November 2019 after obtaining Ethical Clearance (number SDUMC/KLL/IEC/40/2018-2019).
Sample size calculation: Sample size was estimated based on the final scores for two methods, BC-PC and apheresis-PC as per the study done by Mallhi RS et al., in the year 2015 [4] based on the following formula.
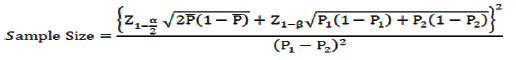
Where,
P1: Proportion of first group
P2: Proportion of second group
α: Confidence Interval (95%)
1-β: Power (80%)
A difference of 38% (as per study by Mallhi RS et al., was observed in getting an excellent result with 95% Confidence Interval (CI) and 80% power [4]. In the present study, to obtain a difference of atleast 25% of excellent result, a sample size of 52 was obtained in each group (PRP-PC: 52, BC- PC:52 and apheresis PC:52). Total sample size was 52 x 3 =156.
Inclusion and exclusion criteria: The inclusion and exclusion of the blood donors were selected as per the Director General of Health Services (DGHS) India criteria [2]. The inclusion criteria for donor’s selection were body weight > 60 to 65 kg, haemoglobin more than 12.5 g/dL, platelet count more than 2.5 lac/mm3. Exclusion criteria were underweight donors, seropositive blood units, insufficient volume, and intake of aspirin/Non steroidal Anti-Inflammatory Drugs/antibiotics prior to three days of donation and haemodiluted samples. This was done to minimise the confounding effect of donor related variables on the comparative analysis of PRP versus BC-PC versus apheresis units.
The donor details such as age, sex, occupation, detailed medical history that includes habits, drug history, postmedical illness and physical examination of donors for general health, blood investigations including Complete Blood Count (CBC) and screening for Transfusion-Transmitted Infections (TTI) were done. Subsequently, the donors were counseled and explained regarding the blood donation and apheresis procedure and also the possible adverse effects. Written consent was obtained for the above procedure. Examination of donors was done as per the standard operating procedure [2]. The appropriate phlebotomy site was examined and adequate arm disinfection was done to mitigate risk of bacterial contamination of PCs.
Each of the PC unit prepared by different methods were assessed as per the recommended DGHS quality norms [2]. This include: (i) Platelet concentrate volume; (ii) Swirling; (iii) Platelet count/bag; (iv) White Blood Cell (WBC) count/bag; (v) pH changes; (vi) Metabolic changes like pO2, pCO2, HCO3; (vii) Mean Platelet Volume (MPV); (viii) Platelet distribution width (PDW); (ix) Platelet Large Cell Ratio (PLCR), respectively.
In this study, the quality of PCs prepared by different methods was analysed as per the recommended DGHS quality norms [Table/Fig-1] [2].
Criteria for quality assessment of Platelet Rich Plasma-Platelet concentrates (PRP-PC), Buffy Coat-Platelet Concentrate (BC-PC) and Apheresis-Platelet Concentrate (Apheresis-PC), according to DGHS technical manual, 2nd Edition [2].
| Quality parameters | PRP-PC | BC-PC | Apheresis-PC |
|---|
| Volume (mL) | 40-70 | 70-90 | 200-300 |
| Swirling | Present | Present | Present |
| Platelet count/bag (in 75% of unit tested) | >5.5×1010 | >5.5×1010 | >3×1011 |
| White blood cells count/bag | 5.5×107 - 5×108 | 5.5×107-5×108 | <5×106 |
| pH (at the end of maximum days of storage in 100% of units tested) | >6.0 | >6.0 | >6.0 |
| Red blood cells (in 100% of units tested) | Traces to 0.5 mL | Traces to 0.5 mL | Traces to 0.5 mL |
| Bacteriological examination (in 100% of units tested) | Sterile | Sterile | Sterile |
Methods
Platelet concentrate volume: Estimation of PC volume is essential because it reflects the amount of plasma present which, inturn, acts as a buffering agent and helps in pH maintenance. Volume of each unit is determined by calculating with the following formula:
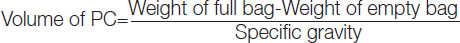
The specific gravity includes (1.053 for whole blood, 1.03 for PRP-PC and 1.06 for BC-PC, respectively).
Swirling: Swirling is a simple procedure which can be routinely used to assess platelet morphology. It is done by holding the PC unit against light at 1 hour, 24 hours and 72 hours, respectively and are given the scoring as follows:
Score (0)- homogenous turbid and is not changed with pressure,
Score (1)- homogeneous swirling only in some part of the bag and is not clear,
Score (2)- clear homogeneous swirling in all part of the bag,
Score (3)- very clear homogeneous swirling in all parts of the bag [5].
pH changes: Maintaining pH of PC >6.0 is one of the important quality parameter. As the storage period of the PC increases, the pH of the PCs correspondingly decreases which results in change of platelet shape from disc to sphere. This alteration in shape results in swelling and loss of function of PCs [6]. The pH was evaluated at the end of the maximum days of storage by the use of a calibrated portable pH meter (OAKTON pH 700, Oatkon instruments, IL, USA), using Standard Operating Procedure (SOP).
Metabolic changes: Estimation of metabolic changes is essential because pH maintenance of PCs is dependent on pO2, pCO2, HCO3, respectively. As the duration of PC storage increased, the continued metabolic activity caused an increase in the mean pO2 and decrease in mean HCO3- levels resulting in a significant fall in pH affecting the function, survival and morphology of platelets [7]. The metabolic parameters were analysed using Arterial Blood Gas (ABG) analyser (Siemens corporation Germany Ltd.,) to assess the platelet viability.
White Blood Cell (WBC) count per bag: Performing of WBC Count in stored PCs is required as the high WBC count in PC causes all immune reaction [8]. Febrile Non Haemolytic Transfusion Reactions (FNHTR) and Graft Versus Host Disease (GVHD) in transfused individuals [9]. The WBC count per bag was performed multiplying WBC count/μl with whole blood volume using a routinely calibrated automated haematology analyser, (Sysmex XE 2100 analyser Sysmex Corporation, Japan).
Platelet count per bag: The platelet count is dependent upon different methodologies used for platelet preparation. Generally, PRP-PCs have a lesser platelet count as compared to BC-PC and SDPs. This is because the centrifugation conditions used with PRP method results in an average of 21% plasma and 19% of the platelets remaining confined to the infranatant RBCs [10]. The platelet count per bag was done multiplying platelet count/μl with product volume using a routinely calibrated automated haematology analyser, (Sysmex XE 2100 analyser Sysmex Corporation, Japan).
Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), Plateletcrit (PCT): Platelet parameters such as MPV, PDW and PCT are important morphological indices which reflects storage related activation changes over a period of days. These platelet indices helps in assessing the platelet morphology and the onset of PSL [11]. MPV is a measure of thrombocyte volume which is expressed in femtoliters (FL). The normal range is 7.2 to 11.7 fL [2]. PDW is indicator of volume variability in platelets size which is expressed as percentage (%). The normal range is 8.3 to 56.6% [2]. PCT is indicator of volume occupied by platelets in the blood which is expressed as percentage (%). The normal range is 0.22 to 0.24% [2]. This indices were analysed using a routinely calibrated automated haematology analyser, (Sysmex XE 2100 analyser Sysmex corporation, Japan).
Red Blood Cells (RBC) count per bag: The presence of RBC during PC preparation is absolutely undesirable and is considered as a contamination which can cause serious adverse effects to the recipients. RBC estimation was done using a routinely calibrated automated haematology analyser, (Sysmex XE 2100 analyser SysmexCorporation, Japan). The permissible range of RBC Count per bag is from traces to 0.5 mL [2].
Bacteriological examination: As PCs are stored in room temperature they are highly susceptible for bacterial contamination. Hence, bacteriological examination using an automated system (BACT ALERT) was performed to rule out bacterial contamination. As per the DGHS Criteria no bacterial contamination is acceptable and all the PC units should be sterile [2].
Cumulative quality assessment scoring: Assessment score was given according to the number of quality parameters achieved by each PC unit. This scoring was based on the five quality parameters including swirling, pH, volume, WBC count/bag and platelet count/bag, as per DGHS criteria [2]. For example a score of 5 or 4 was given to only those PC units which attained 5 or 4 recommended quality control parameters.
Statistical Analysis
All data were expressed as Mean±SD. Data was analysed using appropriate statistical technique. Statistical comparison by using student t-test. The p-value less than 0.05 were considered statistically significant. Statistical Package for the Social Sciences (SPSS) software version 23.0 was used for analysis.
Results
The quality parameters of each PC obtained in this study has been explained below:
Swirling: The maximum number of PCs having a score of 3 was seen in SDP i.e., 52,100%. SDP units had better swirling score when compared to PRP-PC (45, 86.5%) and BC-PC (47, 90.3%) units. The difference was statistically significant (p-value<0.001). As swirling phenomenon is a subjective phenomenon authors could not reach a final conclusion on the remaining 12 bags of PC which remained as inconclusive.
pH: All 156 PCs prepared by 3 methods had a pH of >6.0 at end of maximum storage period.
Volume: The mean volume of PRP-PC, BC-PC and SDP units were 53.75±8.85, 73.65±6.34 and 266.83±17.57, respectively and all were within acceptable quality standards [Table/Fig-2].
Comparative analysis of quality parameters.
| Quality parameters | PRP-PC | BC-PC | SDP | p-value |
|---|
| Mean | SD | Mean | SD | Mean | SD |
|---|
| Volume (mL) per bag | 53.75 | 8.85 | 73.65 | 6.34 | 266.83 | 17.57 | 0.0012 |
| WBC count per bag | 10.03 × 107 | 1.35 × 107 | 1.90 × 107 | 1.05 × 107 | 1.08 × 108 | 0.14 × 108 | 0.0032 |
| Platelet count per bag | 3.32 × 1010 | 1.60 × 1010 | 5.52 × 1010 | 3.54 × 1010 | 2.64 × 1011 | 0.46 × 1011 | 0.0046 |
p-value <0.05 considered significant
WBC count/bag: Total 92.3% (48/52) of SDPs had WBC count per bag within the acceptable limits whereas only 86.5% (45/52) BC-PC and 82.7% (43/52) of PRP-PC had WBC count/bag within the acceptable limits [Table/Fig-2].
Platelet count/bag: Platelet count/bag was analysed in all 156 PCs prepared by different methods. Total 5.8% of PRP-PC (3/52) units had platelet count <55,000 cells which failed to meet required quality criteria. While 100% of the BC-PC and apheresis-PC units met the desired quality control criteria with respect to platelet count/bag. [Table/Fig-2].
Scoring: Scoring was done based on the five quality parameters discussed above: swirling, pH, volume, WBC count/bag and platelet count/bag, as per DGHS criteria [2]. An 11.53% of PRP-PC, 30.76% of BC-PC and 34.6% SDP units had a score of 5, whereas 48.07% of PRP-PC, 48.07% of BC-PC and 50% of SDP units had a score of 4 indicating that BC-PC units were in comparison with SDP units respectively. This was done to initiate objectivity and reproducibility of assessment standard of various quality parameters of PC prepared by different methods [Table/Fig-3].
Scores for the three methods of PC preparation evaluated for the quality parameters.
| Score | PRP-PC | BC-PC | SDP |
|---|
| n | % | n | % | n | % |
|---|
| 5 | 6 | 11.53 | 16 | 30.76 | 18 | 34.61 |
| 4 | 25 | 48.07 | 25 | 48.07 | 26 | 50 |
| 3 | 15 | 28.8 | 11 | 21.11 | 8 | 15.3 |
| 2 | 5 | 9.6 | - | - | - | - |
| 1 | 1 | 1.9 | - | - | - | - |
Platelet Indices: A 48/52 (92.3%) of SDP units had all the platelet indices (MPV, PDW, PCT) within the acceptable limits whereas 43/52 (82.7%) of BC-PC units and only 38/52 (73.1%) of PRP-PC units had platelet indices within the acceptable limits [Table/Fig-3].
ABG analysis: Metabolic indicators were maintained in all the SDP (52/52) and BC-PC (52/52) units whereas only 37/52 (73.1%) of PRP-PC could maintain the metabolic parameters within the acceptable limits.
Bacteriological examination: All the (52/52) of SDPs, (52/52) of BC-PCs and (52/52) of PRP-PC were sterile during the bacteriological examination.
RBC contamination: None of the units (0/52) of SDPs, (0/52) BC-PC, (0/52) of PRP-PC had any evidence of RBC contamination.
Discussion
Current emphasis on accreditation of blood banks has led to greater interest on quality aspects of Whole Blood (WB) derived blood components and their efficacy in maintaining their quality parameters with respect to various regulatory bodies such as DGHS [2]. Our study was one such step in that direction to assess the various quality parameters of PCs prepared by different methodologies and also their degree of compliance with the requirements of regulatory bodies. In the present study, platelet indices MPV, PDW and PLCR had maintained within normal range in maximum number of SDP units followed by BC-PC and the least being PRP-PC units indicating the morphology and function was well maintained in SDP and BC-PC units. The results obtained by Dumont LJ et al., was in comparison to present study with respect to platelet indices in PRP-PC and BC-PC units [11]. Similar to this study, the study conducted by Singh RP et al., the mean volume of PRP-PC, BC-PC and SDPs (Apheresis-PC) was 62.30±22.68 ml, 68.81±22.95 mL [12] and 214.05±9.91 ml, respectively and according to Ashish J et al., the mean volume of PRP-PC, BC-PC and SDPs (Apheresis-PC) was 65.02±6.36, 81.50±6.5, 294.6±8.56 respectively [13]. This highlights the need for further modification and standardisation of the procedures regarding PC-collection preparation and storage without adversely affecting the quality parameters. In addition Murphy S et al., has observed that with the current practice of using second generation blood bags for collection, preparation and storage of PC the plasma volume can be reduced to a maximum of 30 ml without adversely affecting the platelet morphological and functional integrity include the haemostatic function [14]. The functions of transfused platelets in circulation will be depending on the ex-vivo storage lesions determining platelet functionality and status of recipient. These two factors have astrong influence on platelet survival and post transfusion recovery of platelets [15].
Morphological changes noted in stored platelets have been defined in three categories that are platelet activation, metabolic alterations and platelets enescence. As the platelets were stored for longer duration, it showed high levels of platelet activation i.e., about 30% [16]. Parameters such as MPV and PDW are important morphological indices which reflects storage related activation changes over a period of days. This changes are more in PRP-PC as compared to BC-PC and SDP [16]. PC’s were prepared by three different methods namely PRP method, BC method and apheresis method, which causes variability in quality control of PC. Hence, quality assessment plays an important role in evaluating the in-vitro function and viability of platelets transfused and post transfusion platelet recovery in recipients. This assessment of quality helps in providing assurance that PCs prepared are within the recommended specifications [17]. This study highlights the possibility of using platelets morphological parameters such as MPV, PDW and PCT as useful screening test to detect platelet activation because they are simple, convenient and cost effective quality indicators for routine use in resource constraint setup like us. In India, blood banks located in certain regions of the country are fully automated and have complete accreditation of their services which can meet all the quality parameters of AABB and DGHS. However, there are many blood banks located attier II cities and rural areas were automation and accreditation are non existent and they fail to meet the mandatory quality parameters of AABB and DGHS.
In India, there are limited use of BC-PC and Apheresis platelets but PR-PC is widely used which has relatively poor quality parameters [18]. In order to ensure uniformity, objectivity and reproducibility of assessment of various quality parameters of PC prepared by different methodologies a scoring was done based on the five quality parameters such as: swirling, pH, volume, WBC count/bag and platelet count/bag, as per DGHS criteria [Table/Fig-4] [2]. In the present study, it was concluded that SDP units were superior followed by PRP-PC and the least was BC-PC units.
Comparative study of scoring of PC units prepared by different methods with other studies.
| Scores | Score 5 n(%) | Score 4 n (%) |
|---|
| PRP-PC | BC-PC | SDP | PRP-PC | BC-PC | SDP |
|---|
| Mallhi R S et al., (n=100) [4](2015). | | 26 (52) | 45 (90) | | 17 (34) | 4 (8) |
| Talukdar B et al. (n=105) [8] (2017) | 1 (2.5) | 5 (12.5) | 6 (24) | 30 (75) | 34 (85) | 17 (68) |
| Trivedi MP et al.. (n=119) [19] (2017) | 18 (50) | 19 (32.7) | 17 (68) | 12 (33.34) | 28 (48.3) | 6 (24) |
| Present study (n=156) | 6 (11.5) | 16 (30.76) | 18 (34.61) | 25 (48.07) | 25 (48.07) | 26 (50) |
Mean Volume of BC-PC and SDP units this study was 73.65±6.34 and 266.8±17.57 respectively which was comparable to study done by Mallhi RS et al., [4] which showed mean volume of 74.33±9.61 for BC-PC and 269.13 24.1 for SDP unit respectively. Mean Volume PRP-PC in this study was 53.75±8.8 which was in comparison to study done by Raturi M et al., which demonstrated value of 53.6±7.0 [Table/Fig-5] [9].
Comparison of PCs with respect to mean volume/bag (mL) with various studies.
| Methods of preparation | Singh RP et al., [12] 2009 | Ashish J et al., [13] 2015 | Mallhi RS et al., [4] 2015 | Talukdar B et al., [8] 2017 | Raturi M et al., [9] 2017 | Present study |
|---|
| PRP-PC | 62.3±22.6 | 65.02±6.36 | - | 59.4±10.2 | 53.6±7.0 | 53.75±8.85 |
| BC-PC | 68.8±22.95 | 81.50±6.5 | 74.33±9.61 | 63.7±14.3 | 59.3±8.9 | 73.65±6.34 |
| SDP | 214.05±9.91 | 294.6±8.56 | 269.13±24.1 | 209.8±34.6 | - | 266.8±17.57 |
In the present study, WBC count in PRP-PC was 10.03±1.35×107 which was comparatively higher whereas the WBC count in SDP was 1.08±1.04×108 which was nearly comparable to study done by Talukdar B et al., which showed WBC count in SDP as 1.7±1.1×106 [8]. The WBC count in BC-PC was 1.90±1.05×107 which was also nearly comparable to study done Talukdar B et al., which had a WBC count in BC-PC as 1.7±1.0×107 [Table/Fig-6] [8].
Comparative study of WBC count/bag in PC units prepared by different methods with other studies.
| Methods of preparation | Singh RP et al., [12] 2009 | Mallhi RS et al., [4] 2015 | Ashish J et al., [13] 2015 | Talukdar B et al., [8] 2017 | Raturi M et al., [9] 2017 | Present study |
|---|
| PRP-PC | 4.05±0.48 ×107 | | 5.99±1.44 ×107 | 3.2±2.1 ×107 | 1.73±1.7 ×107 | 10.03±1.35 ×107 |
| BC-PC | 2.08±0.39 ×107 | 2.92±1.2 ×107 | 6.16±0.93 ×107 | 1.7±1.0 ×107 | 1.46±1.2 ×107 | 1.90±1.05 ×107 |
| SDP | 4.8±0.8 ×106 | 3.14±1.3 ×107 | 4.87±0.75 ×106 | 1.7±1.1 ×106 | | 1.08±0.14 ×108 |
The present study proves that SDP is ac apital intensive, technologically advanced technique having high maintenance cost but has superior quality parameters in all respect and hence efforts must be made to improve and standardise the quality parameters of PRP-PC, BC-PC respectively which are more widely used in routine practices particularly with regard to similar rural and resource constraints setup like us which caters to the large section of the population.
Limitation(s)
More studies are needed to further validate the different methods used for PC collection, preparation and storage along with proper emphasis regarding the in-vivo maintenance of quality parameters without compromising blood safety. Hence, authors recommended more such studies to further substantiate the findings and make necessary recommendations regarding quality improvement of PCs.
Conclusion(s)
Quality improvement is never a destination but a journey which has to continuously innovate and evolve in due course to meet new challenges and demand. Therefore measures like accreditation of blood banks, adherence to standards of regulatory bodies such as DGHS, using SOPs, strict maintenance of internal quality controls, regular training of technical personnel along with the development “Quality management system” coupled with blood bank automation will go a long way in devising quality parameters in whole blood derived blood products such as PCs prepared by different methodologies.
p-value <0.05 considered significant