Introduction
Exposure to various drugs and chemicals lead to oxidative stress. Carbon Tetrachloride (CCl4) produces rise in oxidative stress leading to hepatic damage. The drug Trimetazidine (TMZ) shows hepatoprotective activity but its mechanism is not known. The present study would help in establishing antioxidant activity of TMZ as probable mechanism.
Aim
To evaluate the antioxidant potential of TMZ in CCl4 induced oxidative stress when given prophylactically/therapeutically in rats.
Materials and Methods
An experimental animal study was conducted on 80 adult Wistar rats of either sex (weight-150 to 200 gm) from March 2010 to December 2010 in Bharati Vidyapeeth Medical College, Pune, Maharashtra, India. Randomly, all animals were grouped into 10 equal groups. Group i was normal control (received only water). To induce oxidative stress CCl4 (0.5 mL/kg/d i.p.) was given to all the animals of Group ii to Group x for seven days. The TMZ was given in two doses, TMZ1 (5 mg/kg orally for Group iii and vii) and TMZ2 (10 mg/kg orally for Group iv and viii). Positive standard control (Group v and Group ix) received Liv.52 (1 mL/kg orally). Group vi and Group x received combination of TMZ1 (5 mg/kg orally)+Liv.52 (1 mL/kg orally). Drug treatment was given to animals in group iii, iv, v and vi for 1-14 days (preventive group) and in group vii, viii, ix and x from day 8 to day 14 (therapeutic group). On 15th day, rats were sacrificed and dissected for collection of liver. Part of the livers was homogenised to assess oxidative stress marker enzymes Malondialdehyde (MDA), Superoxide Dismutase (SOD) spectrophotometrically. Statistical analysis was done with one-way Analysis of Variance (ANOVA) followed by post-hoc analysis (Dunnett’s test) using GraphPad Prism 5.0 software.
Results
Trimetazidine (5 mg/kg and 10 mg/kg) significantly reduced MDA levels and increased SOD levels when compared with CCl4 treated group suggested antioxidant activity. Combined administration of Liv.52 and TMZ1 also reduced oxidative stress and increased antioxidant activity.
Conclusion
Results of the present study suggested that increased oxidative stress was significantly attenuated by drug TMZ in dose dependant manner when compared with the CCl4 group. The antioxidant potential of prophylactic and therapeutic administration of TMZ was comparable. The increased antioxidant effect by Liv.52+TMZ1 combination was only due to the additive antioxidant effects of Liv.52 and TMZ or any other mechanism was involved, needs to be further evaluated.
Introduction
Oxygen metabolism derives highly chemically reactive molecules such as Reactive Oxygen Species (ROS). Free radicals of ROS, such as the Superoxide Anion (O2-) and Hydroxyl Radicals (OH-) etc., or non radical forms, such as Hydrogen Peroxide (H2O2) can be found. Incomplete reduction of oxygen during respiration or metabolic processes carried out by enzymes like nitric oxidase synthase, nicotinamide adenine dinucleotide phosphate oxidase, cyclo-oxygenase, or SOD, can generate ROS [1].
Drug induced liver injury leads to abnormalities in liver function which is caused by various drugs, herbs, or other xenobiotics. The spectrum of liver damage is variable and broad, from acute hepatitis to chronic hepatitis, fatty liver or steatohepatitis, vascular damage, liver cirrhosis, and even hepatic tumours [2]. Over production of ROS and depletion of Adenosine Triphosphate (ATP) [3] results from inhibition of the mitochondrial respiratory chain. Imbalance between ROS production and elimination through antioxidant mechanisms, causing their accumulation may be produced in chronic liver disease, increased inflammation, enzymatic deregulation and mitochondrial damage. This eventually leads to a state of oxidative stress. In this process, ROS participate in the uncontrolled oxidation of lipids, proteins and Deoxyribonucleic Acid (DNA) molecules, therefore causing severe cell damage. Thus, ROS cause parenchymal injury, even inducing further inflammation and progression of liver disease [4]. ROS, at low levels, take part in many physiological processes, including intracellular messaging, regulation of cell growth, differentiation, and apoptosis. Even under non pathological conditions, the liver is often exposed to toxic substances present in portal blood, causing an increase in ROS generation. For this reason, the antioxidant response in the liver is essential and highly regulated to ensure homeostasis [5].
Exposure to chemicals also leads to hepatic damage. Excessive intake of Ethanol, Carbon Tetrachloride (CCl4), iron etc. causing accumulation of free radicals in the body are responsible for impairment of various functions of the liver, such as fatty acid metabolism, protein synthesis, detoxification etc. [6]. However, liver problems are on the rise. It is reported that the annual incidence of drug induced liver injury is between 10 and 15 per 10,000 to 100,000 persons [7]. Till today, there is no standard drug for hepatic disorders. Liv.52, one of the herbal preparation is commonly used as standard control in various experimental models of hepatic damage and it is being also used by modern medicine practitioners [8].
One of the add on drugs in the management of angina is TMZ [9]. The exact mechanism of action of TMZ is not known. However, it is said that it inhibits mitochondrial long chain 3-ketoacyl-coA-thiolase (LC 3KAT). It reduces fatty acid metabolism and increases glucose metabolism. It also reduces O• free radical induced membrane damage [10].
The drug TMZ has a protective effect on intrahepatic cholestasis caused by carmustine [11]. It also has antioxidant status in trinitrobenzenesulfonic acid-induced chronic colitis [12] and ethanol and acetic acid induced colitis [13]. It is also known for its hepatoprotective action when given prophylactically [14] as well as therapeutically [15] in CCl4 induced hepatic damage/oxidative stress in albino rats. However, mechanism of action of its hepatoprotective action is still unknown. The TMZ is also known to have antioxidant activity and this present study was planned to evaluate the antioxidant potential of TMZ in CCl4 induced oxidative stress in liver, when it was given prophylactically as well as therapeutically in albino rats.
Materials and Methods
The present experimental animal study was conducted from March 2010 to December 2010 in Bharati Vidyapeeth Medical College, Pune, Maharashtra, India. Before initiating the study, prior approval of Institutional Animal Ethics Committee (Approval Letter No. IAEC/BVDUMC/2009-2010 dated 2/9/2009) was taken. During entire study, guidelines formulated by Committee for the purpose of control and supervision of experiments on animals were followed [16].
Eighty Wistar rats of either sex weighing 150 to 200 gm were included for the study. The animals were housed in clean, sterile polypropylene cages. Standard conditions of 12 hour light/12 hour dark cycle, humidity of 50% and temperature of 25°C were followed. The standard pelleted diet (Pranav Agro Industries Ltd., Pune, Maharashtra, India) was fed and aquaguard water ad libitum was provided to all animals.
Drugs and Chemicals
1) Trimetazidine (TMZ): The TMZ Dihydrochloride was available in the brand name of Trivedon-20 tablets (20 mg/tablet), manufactured by Cipla Ltd., Solan, HP, India. Tablets were obtained from the medical store which was water soluble. Minimum {TMZ1 (5 mg/kg)} and maximum {TMZ2 (10 mg/kg)} single daily dose of TMZ was decided. Drugs were administered orally.
2) Carbon Tetrachloride (CCl4): It was Laboratory Reagent Grade, Mol.Wt.153.82, and was obtained from Central Drug House Private Ltd, New Delhi. The CCl4, 0.5 mL/kg/d i.p. for 7 days was used to induce oxidative stress [17].
3) Liv.52: It is known antioxidant used as standard control which has many herbs effective against liver disorders. Drug was obtained from The Himalaya Drug Company, Solan, HP, India. It was given orally in the dose of 1 mL/kg orally [8].
All the animals were randomly grouped into 10 equal groups. Group i was normal control (received only water). To induce oxidative stress CCl4 (0.5 mL/kg/d i.p.) was given to all the animals of Group ii to Group x for 7 days. The TMZ was given in two doses, TMZ1 (5 mg/kg orally for Group iii and vii) and TMZ2 (10 mg/kg orally for Group iv and viii). Positive standard control (Group v and ix) received Liv.52 (1 mL/kg orally). Group vi and x received combination of TMZ1 (5 mg/kg orally)+Liv.52 (1 mL/kg orally).
Drug treatment was given to animals in group iii, iv, v and vi for 1-14 days (preventive group) and in group vii, viii, ix and x from day 8 to day 14 (therapeutic group) as shown in [Table/Fig-1]. This was done to compare antioxidant potential of prophylactic and therapeutic administration of TMZ.
Animal groups with drugs, dosing and treatment duration.
| Group | Drug treatment | Dose | Duration |
|---|
| i | Normal/Distilled water control | Distilled water | Day 1 to Day 14 |
| ii | CCl4 control | 0.5 mL/kg/d i.p. | Day 1 to Day 7 (to animals of all groups except group i) |
| iii | TMZ1 | 5 mg/kg orally | Prophylactic treatment (dosing from day 1-14) |
| iv | TMZ2 | 10 mg/kg orally |
| v | Liv.52 | 1 mL/kg orally |
| vi | TMZ1+Liv.52 | 5 mg/kg+1 mL/kg orally |
| vii | TMZ1 | 5 mg/kg orally | Therapeutic treatment (dosing from day 8-14) |
| viii | TMZ2 | 10 mg/kg orally |
| ix | Liv.52 | 1 mL/kg orally |
| x | TMZ1+Liv.52 | 5 mg/kg+1 mL/kg orally |
Assessment of Oxidative Stress
After the drug treatment, all the animals were sacrificed on 15th day. Livers of all the animals were dissected out. Some part of the liver was used for the antioxidant enzyme assays. Liver tissues were collected in the tissue sample bottles in TC199 media and were taken for assessment of oxidative stress marker enzymes SOD [18] and lipid peroxidation (MDA level) [19] spectrophotometrically. Measurement of lipid peroxidation (MDA level) was considered as marker of oxidative stress whereas SOD indicated antioxidant levels. For preparation of tissue homogenates, motor driven teflon homogeniser was used. The 50 mM Phosphate buffer (pH7.4) was used for tissue homogenates. Remi Industries Ltd., Remi Laboratory Instruments, Cooling Centrifuge, C-24 BL is low volume high speed model in table top design was used to centrifuge tissue homogenates for removal of debris and nuclei. They were centrifuged at 3000 RPM for 10 minutes at 4°C. After centrifugation, the supernatant was stored at -80°C which consists of cytosolic and mitochondrial fractions. Ultraviolet Visible spectrophotometer, Model Lambda 35, manufactured by Perkin Elmer Inc. USA was used for determination of SOD and Lipid peroxidation (MDA level) activities in the supernatant fluid [20]. Malondialdehyde (MDA) as an end product of lipid peroxidation which reacts with thiobarbituric acid producing Thiobarbituric Acid Reactive Substance (TBARS), a pink chromogen, which can be measured spectrophotometrically at 532 nm [21]. The SOD activity was estimated by its capacity to inhibit pyrogallol autoxidation in alkaline medium. This was measured at 420 nm [22].
Statistical Analysis
All the findings were recorded as the mean±SEM. Statistical analysis was done with One-way Analysis of Variance (ANOVA) followed by post-hoc analysis (Dunnett’s test) using GraphPad Prism 5.0 software.
Results
Malondialdehyde (MDA) (nmol/g) and SOD (IU/mg protein) levels of the liver tissue homogenates of different groups were estimated on day 15 [Table/Fig-2,3 and 4]. The data were compared and analysed statistically within the different groups.
Effect of TMZ on lipid peroxidase (MDA-Malondialdehyde level) and Superoxide Dismutase (SOD).
| Groups | MDA (nmol/gm) | p-value | SOD (IU/mg protein) | p-value |
|---|
| i-Distilled water control | 6.05±0.52 | | 1.23±0.15 | |
| ii- CCl4 Control | 11.9±1.21≠ | 0.0007 | 0.79±0.07≠ | 0.0371 |
| Iii●- CCl4+TMZ1 | 9.34±0.70** | 0.0038 | 1.12±0.08 | 0.0679 |
| iv●- CCl4+TMZ2 | 8.37±0.30*** | 0.0006 | 1.21±0.10* | 0.0366 |
| v●- CCl4+Liv.52 | 7.43±0.25*** | 0.0005 | 1.36±0.09*** | 0.0007 |
| vi●- CCl4+Liv.52+TMZ1 | 7.28±0.28*** | 0.0009 | 1.44±0.10*** | 0.0002 |
| vii■ - CCl4+TMZ1 | 9.86±0.36* | 0.0373 | 1.20±0.10* | 0.0227 |
| viii■- CCl4+TMZ2 | 9.17±0.16** | 0.0029 | 1.19±0.13* | 0.0416 |
| ix■- CCl4+Liv.52 | 8.43±0.23*** | 0.0007 | 1.19±0.10* | 0.0263 |
| x■- CCl4+Liv.52+TMZ1 | 6.94±0.22*** | 0.0003 | 1.23±0.08** | 0.0070 |
Values indicate Mean±SEM; #means p-value <0.05, ##means p-value <0.001 as compared to normal/vehicle control group; *p-value <0.05, **p-value <0.01, ***p-value <0.001 in comparison with CCl4 control (One-way ANOVA followed by post-hoc Dunnett’s test)
●-Prophylactic dose, ■-Therapeutic dose
MDA: Malondialdehyde; SOD: Superoxide dismutase; CCL4: Carbon tetrachloride; TMZ: Trimetazidine
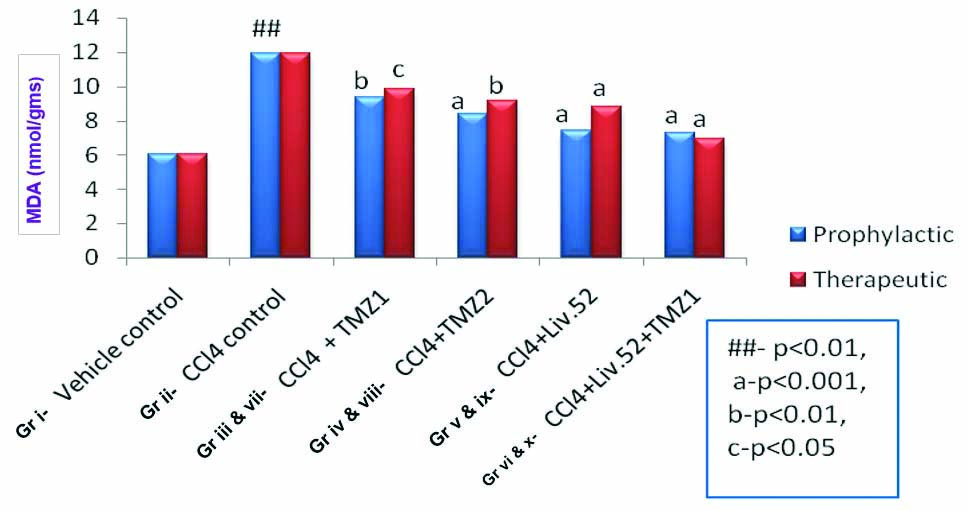
Effects of TMZ, Liv.52 and TMZ+Liv.52 on MDA in CCl4 induced oxidative stress in rats.
(Gr=group)
## means p-value <0.01 as compared to normal/vehicle control group.
a-p-value <0.001, b-p-value <0.01, c-p-value <0.05 in comparison with CCl4 control
(One-way ANOVA followed by post-hoc Dunnett’s test)
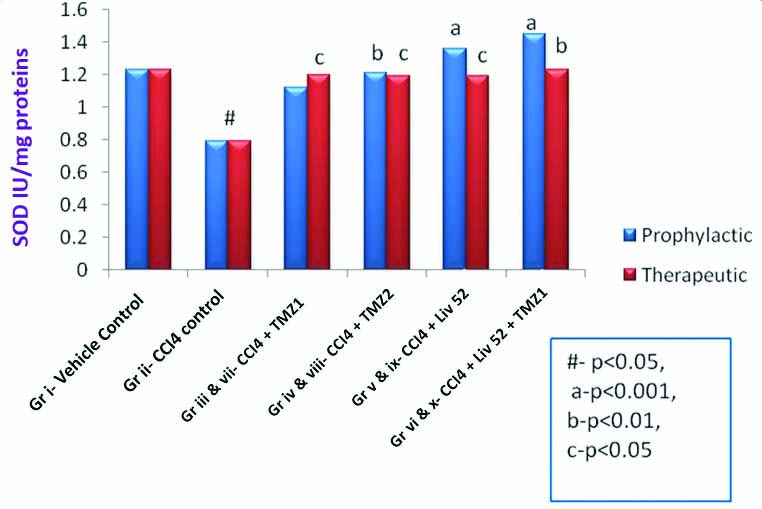
Effects of TMZ, Liv.52 and TMZ+Liv.52 on SOD in CCl4 induced oxidative stress in rats.
# means p<0.05 as compared to normal/vehicle control group.
a-p<0.001, b-p<0.01, c-p<0.05 in comparison with CCl4 control
(One-way ANOVA followed by post-hoc Dunnett’s test)
[Table/Fig-2] shows significant increase (p-value <0.05) in MDA levels by CCl4 (group ii) which suggests definite increase in level of oxidative stress. The TMZ (in both the doses) (group iii, iv and vii, viii), Liv.52 (group v and ix) and TMZ1+Liv.52 (group vi and x) produced significant reduction (p-value <0.05 to p-value <0.001) in MDA (oxidative stress) when administered prophylactically or therapeutically [Table/Fig-3]. There was no statistically significant difference observed when prophylactic and therapeutic treatment groups were compared with each other respectively. It means TMZ1 (group iii and vii), TMZ2 (group iv and viii), Liv.52 (group v and ix) and TMZ1+Liv.52 (group vi and x) showed comparable effects when administered prophylactically as well as therapeutically.
[Table/Fig-2] also shows significant increase (p-value <0.05 to p-value <0.001) in SOD by TMZ (in both the doses) (group iv and vii, viii), Liv.52 (group v and ix) and TMZ1+Liv.52 (group vi and x), when administered prophylactically or therapeutically, indicating antioxidant activity of TMZ as well as Liv.52 [Table/Fig-4]. Antioxidant levels (SOD) when compared between prophylactic and therapeutic groups, did not show any statistically significant difference. It means TMZ1 (group iii and vii), TMZ2 (group iv and viii), Liv.52 (group v and ix) and TMZ1+Liv.52 (group vi and x) showed comparable effects when administered prophylactically as well as therapeutically.
Discussion
Carbon Tetrachloride (CCl4), one of the hepatotoxic agent increased oxidative stress in animals. The drug TMZ had reduced this increased oxidative stress in dose dependant manner when compared with the CCl4 group. Combined administration of Liv.52 and TMZ1 (group vi and x) also reduced oxidative stress and increased antioxidant activity. Our study also showed that antioxidant potential of prophylactic and therapeutic administration of TMZ was comparable.
The liver plays a central role in metabolic homeostasis because it is liable for the metabolism, synthesis, storage and redistribution of nutrients, carbohydrates, fats and vitamins. The main detoxifying organ of the body is liver, which removes wastes and xenobiotics by metabolic conversion and biliary excretion [23].
Oxidative stress to liver occurs by many mechanisms. Toxic substances/pollutants could cause oxidative stress. The CCl4 mediated oxidative stress is one of the common experimental models for hepatic stress. The CCl4 gets deposited in hepatic parenchymal cells. It forms trichloromethyl free radical (CCl3•) when it is metabolically activated by cytochrome P-450 dependent mono-oxygenase. This free radical then alkylates cellular proteins and other macromolecules. This free radical also acts on polyunsaturated fatty acid in the presence of oxygen which in turn forms lipid peroxidase. These two actions lead to liver damage by producing oxidative stress [5]. For prevention and neutralisation of free radical induced damage, our body has an effective defence mechanism. It mainly consists of some of the endogenous antioxidant enzymes, such as SOD, Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione Reductase (GR) etc. However, oxidative stress develops, when there occur imbalance between production of ROS and loss of antioxidant defence mechanism. Rise in oxidative stress, thus developed, produce a series of events which ultimately deregulates the cellular functions leading to cellular damage. Such cellular damage may be partially or totally alleviated by any compound having antioxidant properties whether natural or synthetic [24].
The hepatic oxidative stress stimulated by CCl4 may be reduced by large number of plants, especially medicinal plants. Feng Y et al., and Ye X et al., had studied antioxidant mechanism of Coptidis rhizome and its bioactive compound berberine to relieve CCl4-induced oxidative stress and hepatic damage [24,26]. Vuda M et al., observed antioxidant activity of aqueous extract of Hybanthus enneaspermus in reducing CCl4-induced oxidative stress [27]. Kalegari M et al., also noted antioxidant potential of Rourea induta Planch (Connaraceae) [28]. Similar kind of changes in oxidative stress and antioxidant activity were reported by other researchers for methanolic extract of flowers of Nerium oleander [29], Phyllanthus emblica L. Bark Extract [30], Aconogonon alpinum (All.) Schur roots, Fruit Rind Extract of Garcinia dulcis (Roxburgh) Kurz, red seaweeds of Gracilaria Edulis and Hypnea Valentiae [30-33]. Borole K et al., studied antioxidant potential of malotilate in ethanol induced hepatic dysfunction in Sprague Dawley rats [20].
Mate V et al., evaluated the hepatoprotective activity of TMZ. So, further studies were carried out to explore its mechanism of action [14,15]. TMZ also is reported to have antioxidant property [12]. So, experimental evaluation of TMZ was planned to find out whether TMZ has antioxidant action. Also comparative evaluation of antioxidant profile of TMZ in prophylactic treatment and therapeutic treatment was planned. Sharma M et al., have demonstrated that hepatoprotective effect of Liv.52 was due to its antioxidant activity [34].
The convenient index for determining the extent of the peroxidation reaction is MDA which is formed from the breakdown of Poly Unsaturated Fatty Acids. It reacts with thiobarbituric acid to give a TBARS, i.e., TBARS. Estimation of MDA levels, therefore, is used as indicator of lipid peroxidation. Increased levels of MDA in CCl4 treated group (group ii) indicated that CCl4 increases lipid peroxidation i.e., oxidative stress [35]. The significant decline in MDA levels in the liver of TMZ1 (group iii and vii) and TMZ2 (group iv and viii) treated rats indicated reduced oxidative stress in dose dependant manner in both prophylactic as well as therapeutic groups.
Girgin F et al., discussed effectiveness of TMZ in intrahepatic cholestasis caused by carmustine [11]. The TMZ was also effective in colitis induced by different mechanisms primarily by its antioxidant action [12,13]. The TMZ is used clinically as an add-on drug for chronic prophylaxis of angina pectoris. The mechanism by which it benefits in this condition is inhibition of the mitochondrial long chain 3-ketoacyl-CoA thiolase (LC3-KAT), a key enzyme in fatty acid oxidation and promotion of glucose oxidation in myocardium. It may improve cellular tolerance to ischaemia by modulating myocardial metabolism [10]. The present study demonstrated antioxidant activity of TMZ which can be explored as a mechanism of additional antianginal benefit in humans.
Limitation(s)
Limitation of this study was that we had not studied any other mechanism of reduction of oxidative stress other than antioxidant potential of TMZ.
Conclusion(s)
Increased oxidative stress of liver was significantly reduced in the TMZ treated groups in dose dependant manner when compared with the CCl4 group (group ii). Combined administration of Liv.52 and TMZ1 (group vi and x) also reduced oxidative stress and increased antioxidant activity. Whether this was only due to the additive antioxidant effects of Liv.52 and TMZ or any other mechanism was involved, needs to be further evaluated. The present study also showed that antioxidant potential of prophylactic and therapeutic administration of TMZ was comparable. The antioxidant activity of TMZ can be further explored as a mechanism of additional benefit for various cardiovascular diseases in humans.
Values indicate Mean±SEM; #means p-value <0.05, ##means p-value <0.001 as compared to normal/vehicle control group; *p-value <0.05, **p-value <0.01, ***p-value <0.001 in comparison with CCl4 control (One-way ANOVA followed by post-hoc Dunnett’s test)
●-Prophylactic dose, ■-Therapeutic dose
MDA: Malondialdehyde; SOD: Superoxide dismutase; CCL4: Carbon tetrachloride; TMZ: Trimetazidine
[1]. Brieger K, Schiavone S, Miller FJ Jr, Krause KH, Reactive oxygen species: From health to diseaseSwiss Med Wkly 2012 142:01-14.10.4414/smw.2012.1365922903797 [Google Scholar] [CrossRef] [PubMed]
[2]. Suh JI, Drug-induced liver injuryYeungnam Univ J Med 2020 37(1):02-12.10.12701/yujm.2019.0029731661757 [Google Scholar] [CrossRef] [PubMed]
[3]. Berson A, Renault S, Lettéron P, Robin MA, Fromenty B, Fau D, Uncoupling of rat and human mitochondria: A possible explanation for tacrine-induced liver dysfunctionGastroenterology 1996 110(6):1878-90.10.1053/gast.1996.v110.pm89644148964414 [Google Scholar] [CrossRef] [PubMed]
[4]. Nguyen T, Nioi P, Pickett CB, The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stressJ Biol Chem 2009 284(20):13291-95.10.1074/jbc.R90001020019182219 [Google Scholar] [CrossRef] [PubMed]
[5]. Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, The role of oxidative stress and antioxidants in liver diseasesInt J Mol Sci 2015 16(11):26087-124.10.3390/ijms16112594226540040 [Google Scholar] [CrossRef] [PubMed]
[6]. David S, Hamilton JP, Drug-induced liver injuryUS Gastroenterol Hepatol Rev 2010 6:73-80. [Google Scholar]
[7]. Ahmad J, Odin JA, Epidemiology and genetic risk factors of drug hepatotoxicityClin Liver Dis 2017 21(1):55-72.10.1016/j.cld.2016.08.00427842775 [Google Scholar] [CrossRef] [PubMed]
[8]. Sisodia SS, Bhatnagar M, Hepatoprotective activity of Eugenia jambolana Lam.in carbon tetrachloride treated ratsIndian J Pharmacol 2009 41(1):23-27.10.4103/0253-7613.4888820177577 [Google Scholar] [CrossRef] [PubMed]
[9]. Sweatman SC, Martindale: the complete drug reference 2017 39th edLondonPharmaceutical Press:1018 [Google Scholar]
[10]. Tripathi KD, Essentials of Medical pharmacology 2019 8th edNew DelhiJaypee publication:599 [Google Scholar]
[11]. Girgin F, Tuzun S, Demir A, Kuralay F, Ozutemiz O, Tanyalcin T, The effect of trimetazidine on intrahepatic cholestasis caused by carmustine in ratsExp Toxicol Pathol 1999 51(4-5):326-29.10.1016/S0940-2993(99)80015-3 [Google Scholar] [CrossRef]
[12]. Girgin F, Karaoglu O, Erkuş M, Tuzun S, Ozutemiz O, Dinçer C, Effects of Trimetazidine on oxidant/antioxidant status in trinitrobenzenesulfonic acid induced chronic colitisJ Toxicol Environ Health A 2000 59(8):641-52.10.1080/00984100015663710839497 [Google Scholar] [CrossRef] [PubMed]
[13]. Girgin F, Karaoglu Tuzun Erkus Ozutemiz Dinçer Batur Tanyalçin Effects of trimetazidine in ethanol- and acetic acid-induced colitis: Oxidant/anti-oxidant statusColorectal Dis 1999 1(6):338-46.10.1046/j.1463-1318.1999.00078.x23574598 [Google Scholar] [CrossRef] [PubMed]
[14]. Mate V, Pandit V, Wani D, Dhande P, Role of trimetazidine in carbon tetrachloride induced liver damage in ratsInt J Basic Clin Pharmacol 2014 3(1):164-70.10.5455/2319-2003.ijbcp20140221 [Google Scholar] [CrossRef]
[15]. Mate V, Pandit V, Trimetazidine- assessment of therapeutic effects for liver damage in ratsInt J Pharm Bio Sci 2020 11(3):100-04.10.22376/ijpbs.2020.11.3.p100-104 [Google Scholar] [CrossRef]
[16]. Compendium of CPCSEA 2018. Page 1-213. Available from: http://cpcsea.nic.in/guidelines/ Compendium of CPCSEA 2018.pdf. [Last accessed on 2021 August 30] [Google Scholar]
[17]. Maheshwari MU, Rao PG, Antihepatotoxic effect of grape seed oil in ratIndian Journal of Pharmacology 2005 37(3):179-82.10.4103/0253-7613.16216 [Google Scholar] [CrossRef]
[18]. Dahiru D, Obidoa O, Pretreatment of albino rats with aqueous leaf extract of Ziziphus mauritiana protects against alcohol-linduced liver damageTrop J Pharm Res 2007 6(2):705-10.10.4314/tjpr.v6i2.14649 [Google Scholar] [CrossRef]
[19]. Cleasby ME, Dzamko N, Hegarty BD, Cooney GJ, Kraegen EW, Ye JM, Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanismsDiabetes 2004 53(12):3258-66.10.2337/diabetes.53.12.325815561958 [Google Scholar] [CrossRef] [PubMed]
[20]. Borole K, Swami R, Padalkar P, Study of antioxidant potential of malotilate in ethanol induced hepatic dysfunction in Sprague Dawley ratsInt J Basic Clinl Pharmacol 2016 5(2):384-88.10.18203/2319-2003.ijbcp20160749 [Google Scholar] [CrossRef]
[21]. Noeman SA, Hamooda HE, Baalash AA, Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in ratsDiabetol Metab Syndr 2011 3:1710.1186/1758-5996-3-1721812977 [Google Scholar] [CrossRef] [PubMed]
[22]. Marklund S, Marklund G, Involvement of the superoxide anion radical in the oxidation of pyrogallol and a convenient assay for superoxide dismutaseEur J Biochem 1974 47:469-74.10.1111/j.1432-1033.1974.tb03714.x4215654 [Google Scholar] [CrossRef] [PubMed]
[23]. Oliva J, French BA, Qing X, French SW, The identification of stem cells in human liver diseases and hepatocellular carcinomaExp Mol Pathol 2010 88(3):331-40.10.1016/j.yexmp.2010.01.00320080086 [Google Scholar] [CrossRef] [PubMed]
[24]. Bandyopadhyay U, Das D, Banerjee R, Reactive oxygen species: Oxidative damage and pathogenesisCurrent Science 1999 77(5):658-66. [Google Scholar]
[25]. Feng Y, Wang N, Ye X, Li H, Feng Y, Cheung F, Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in ratsJ Ethnopharmacol 2011 138:683-90.10.1016/j.jep.2011.09.03221963555 [Google Scholar] [CrossRef] [PubMed]
[26]. Ye X, Feng Y, Tong Y, Ng KM, Tsao S, Lau GK, Hepatoprotective effects of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in ratsJ Ethnopharmacol 2009 124:130-36.10.1016/j.jep.2009.04.00319536921 [Google Scholar] [CrossRef] [PubMed]
[27]. Vuda M, D’Souza R, Upadhya S, Kumar V, Rao N, Kumar V, Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in ratsExp Toxicol Pathol 2012 64:855-59.10.1016/j.etp.2011.03.00621478003 [Google Scholar] [CrossRef] [PubMed]
[28]. Kalegari M, Gemin CA, Araujo-Silva G, Brito NJ, López JA, Tozetto Sde O, Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female ratsNutrition 2014 30:713-18.10.1016/j.nut.2013.11.00424800671 [Google Scholar] [CrossRef] [PubMed]
[29]. Singhal KG, Gupta GD, Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in ratsAsian Pac J Trop Med 2012 5:677-85.10.1016/S1995-7645(12)60106-0 [Google Scholar] [CrossRef]
[30]. Chaphalkar R, Apte KG, Talekar YP, Ojha SK, Nandave M, Antioxidants of Phyllanthus emblica L. Bark Extract provide Hepatoprotection against Ethanol-Induced Hepatic Damage: A Comparison with SilymarinOxidative Medicine and Cellular Longevity 2017 2017:387604010.1155/2017/387604028168009 [Google Scholar] [CrossRef] [PubMed]
[31]. Muhammad ZK, Muhammad IS, Saqib Z, Gilani SA, Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur rootsOpen Chemistry 2020 18:516-36.10.1515/chem-2020-0062 [Google Scholar] [CrossRef]
[32]. Gogoi N, Gogoi A, Neog B, Baruah D, Singh KD, Antioxidant and hepatoprotective activity of garcinia dulcisPharmacognosy Research 2017 9(3):266-72.10.4103/0974-8490.21033028827968 [Google Scholar] [CrossRef] [PubMed]
[33]. Mahendran S, Maheswari P, Sasikala V, Rubika JJ, Pandiarajan J, In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiaeToxicol Rep 2021 8:1404-11.10.1016/j.toxrep.2021.07.00634295651 [Google Scholar] [CrossRef] [PubMed]
[34]. Sharma M, Pillai KK, Husain SZ, Giri DK, Protective role of propolis against alcohol- carbon tetrachloride- induced hepatotoxicity in ratsIndian J Pharmacol 1997 29:76-81. [Google Scholar]
[35]. Janero DR, Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injuryFree Radic Biol Med 1990 9(6):515-40.10.1016/0891-5849(90)90131-2 [Google Scholar] [CrossRef]