Case Report
A 58-year-old male patient presented to Emergency Medical Services (EMS) Department of a tertiary care hospital at 11:00 pm with an alleged history of accidental consumption of around 10 mL of 5% topical minoxidil solution. The patient had minoxidil drug sample bottles at his residence as his son was a medical representative and those sample bottles were stored in old alcohol bottles for discarding purpose. Patient consumed the drug mistakenly thinking it as an alcohol at 7pm on the day of reporting for hospital. The patient developed giddiness and two episodes of vomiting without chest pain or breathlessness following consumption. Patient was initially taken to local clinic where they found the patient had severe hypotension and was started on dual inotropic support, and referred to tertiary care hospital for further management.
On presentation, the patient was conscious, restless, afebrile with vital signs as pulse 170 beats/min, blood pressure of 50/20 mmHg, respiratory rate of 30 respiration/min. In view of haemodynamic instability, he was given a fluid challenge with intravenous crystalloids and dual inotropes. Later, intravenous (i.v.) fluids were eventually stopped and the patient was kept on continuous inotrope support. Gastric lavage was performed.
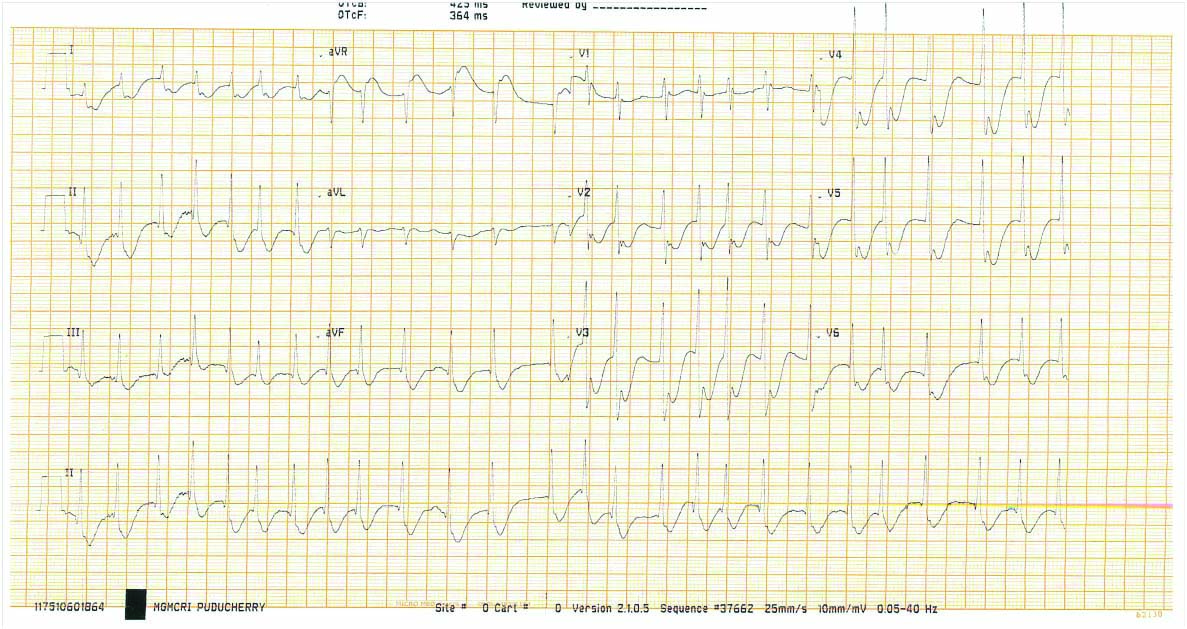
Electrocardiogram (ECG) revealed atrial fibrillation with rapid ventricular rate with incomplete Right Bundle Branch Block (RBBB) pattern [Table/Fig-1]. He had revealed elevated total White Blood Cells (WBC) counts 26,700 cells/mm3, creatinine 1.40 mg/dL, low serum potassium 3.0 mEq/L and normal cardiac enzymes {Creatine phosphokinase-N-acetyl-cystein (CPK-NAC):182U/L, Creatine phosphokinase-MB (CPK-MB):32U/L, Troponin I was negative}. Arterial blood gas analysis showed hypoxia and metabolic acidosis (pH: 7.25, pCO2:38, pO2:61, HCO3:16, SpO2:87%, anion gap: 9.0 mEq/L).
(Day 1 ECG)- A-Fib with RVR and incomplete RBBB pattern.
A-Fib: Atrial fibrillation; RVR: Rapid ventricular response; RBBB: Right bundle branch block; ECG: Electrocardiogram
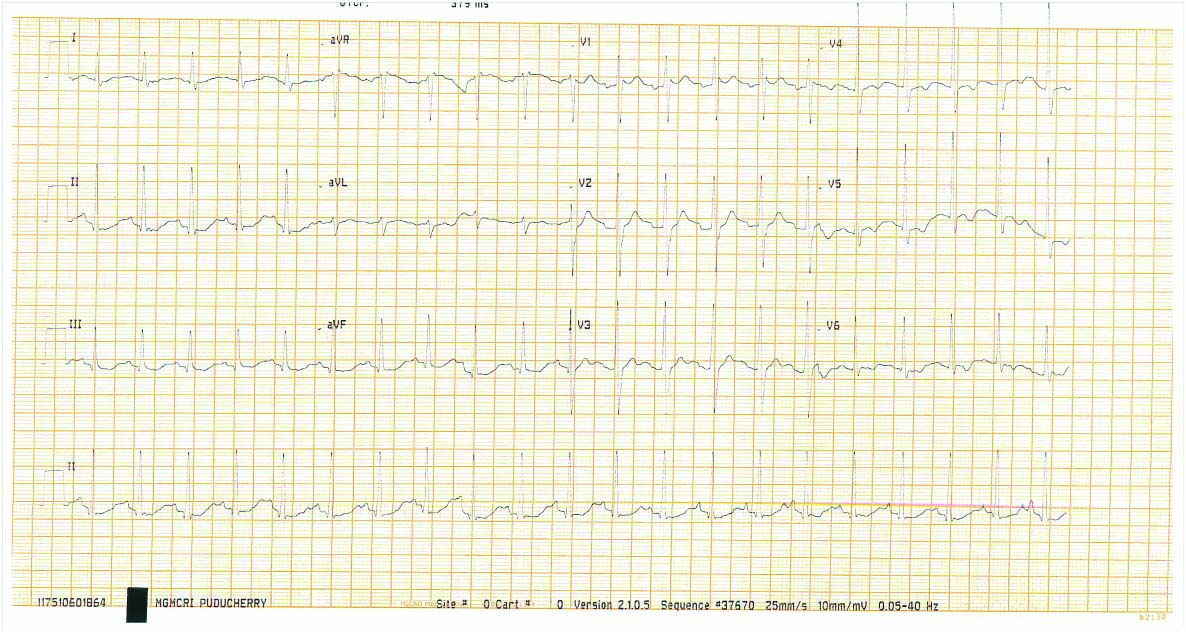
Patient was started on prophylactic antibiotics (Inj.ceftriaxone 1 gm) in view of elevated total counts after sending blood, urine and sputum cultures. Continuous cardiac monitoring was done for two days. Inotropes were titrated according to Mean Arterial Pressure (MAP) (target MAP of 65 mmHg), then gradually tapered and stopped. Serial ECG monitoring done on day 2 [Table/Fig-2] was found to have gradual control in heart rate and stabilisation of ECG. [Table/Fig-3] showed laboratory parameters and the trend of biochemical and haematological parameters during seven days of hospital stay. Total counts gradually came to normal levels after initiation of antibiotics. Renal parameters gradually became normal without any requirement of haemodialysis. Serial measurement of cardiac enzymes repeated after 6 hours (Trop-I:570 ng/L) and 12 hours (Trop-I:178 ng/L) showed a decreasing trend. During hospital stay, patient was found to have high fasting blood sugar (280 mg/dL) and HbA1C (12.2 g%). The patient was thus initiated with split dose insulin, and careful monitoring of blood glucose levels and insulin dose titrated accordingly.
(Day 2 ECG)-Gradual resolution of A-Fib and incomplete RBBB pattern.
ECG: Electrocardiogram
Laboratory parameters during hospital stay of one week.
| S. No. | Lab test | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|
| 1. | Total counts (cells/mm3) | 26700 | 22200 | 15600 | 13400 | 10900 | 11500 |
| 2. | Haemoglobin (gm/dL) | 11.3 | 12.6 | 11.2 | 11.0 | 11.4 | 13.3 |
| 3. | Platelet count (cells/mm3) | 348000 | 387000 | 293000 | 253000 | 285000 | 436000 |
| 4. | Blood urea (mg/dL) | 26 | 31 | 49 | 59 | 55 | - |
| 5. | Creatinine (mg/dL) | 1.40 | 1.25 | 0.99 | 0.93 | 0.84 | - |
| 6. | Sodium (mEq/L) | 139 | 142 | 144 | 140 | 139 | - |
| 7. | Potassium (mEq/L) | 3.0 | 2.9 | 3.7 | 3.5 | 4.1 | - |
| 8. | Chloride (mEq/L) | 114 | 112 | 115 | 109 | 106 | - |
| 9. | Calcium (mg/dL) | 7.9 | - | 7.5 | - | - | - |
| 10. | Magnesium (mg/dL) | 2.4 | - | - | - | - | - |
| 11. | Phosphorous (mg/dL) | 3.9 | - | - | - | - | - |
| 12. | Total protein (gm/dL) | 5.8 | 6.1 | - | 5.3 | - | - |
| 13. | Albumin (gm/dL) | 3.5 | 3.7 | - | 3.1 | - | - |
| 14. | Total bilirubin (mg/dL) | 0.4 | 0.5 | - | 0.8 | - | - |
| 15. | Direct bilirubin (mg/dL) | 0.2 | 0.2 | - | 0.2 | - | - |
| 16. | SGOT (U/L) | 34 | 25 | - | 26 | - | - |
| 17. | SGPT (U/L) | 26 | 21 | - | 23 | - | - |
| 18. | ALT (U/L) | 91 | 81 | - | 72 | - | - |
| 19. | CRP | Negative | - | - | - | - | - |
| 20. | ESR (mm/hr) | 40 | - | - | - | - | - |
| 21. | Ferritin (ng/mL) | 47.5 | - | - | - | - | - |
| 22. | D dimer (ng/mL) | 568 | - | - | - | - | - |
| 23. | Urine Routine | Bacteria-nil | - | - | - | - | - |
| 24. | CPK-NAC (U/L) | 182 | 294 | 216 | - | - | - |
| 25. | CPK-MB (U/L) | 32 | 20 | 12 | - | - | - |
| 26. | Trop-I (ng/L) | Negative | 570 | 178 | - | - | - |
| 27. | PT-INR (sec) | 0.89 | - | - | - | - | - |
| 28. | APTT (sec) | 31.8 | - | - | - | - | - |
| 29. | ABG (mmHg), HCO3 (mEq/L) | pH: 7.25, pC02:38,pO2:61, HCO3:16,SO2:87 | pH: 7.31, pC02:34,pO2:60, HCO3:17,SO2:88 | pH: 7.36, pC02:35,pO2:74, HCO3:19,SO2:94 | pH: 7.46, pC02:31,pO2:66, HCO3:22,SO2:94 | pH: 7.47, pC02:33,pO2:62, HCO3:24,SO2:93 | pH: 7.43, pC02:38,pO2:86, HCO3:25,SO2:97 |
| 30. | Cultures | 1) Urine culture: Sterile2) Blood culture: Sterile3) Sputum culture: Klebsiella Pneumonia- ESBL producer | - | - | - | - | |
SGOT: Serum glutamic-oxalacetic transaminase; SGPT: Serum glutamic-pyruvic transaminase; ALT: Alanine aminotransferase; CRP: C-reactive protein test; ESR: Erythrocyte sedimentation rate;
PT/INR: Prothrombin time and international normalised ratio; APTT: Activated partial thromboplastin time; ABG: Arterial blood gases; CPK-NAC: Creatine phosphokinase-N-acetyl-cystein; CPK-MB: Creatine phosphokinase -MB; Troponin- I
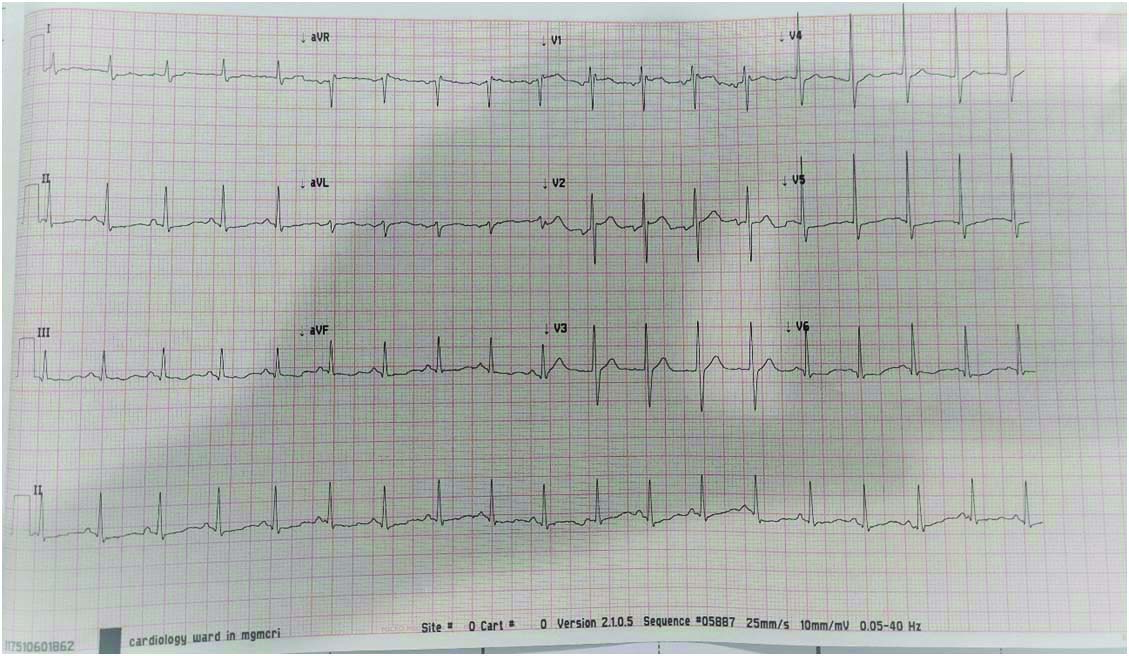
During the hospital stay on day 3, patient had breathlessness, tachypnoea, sudden desaturation (SpO2 -84%) and auscultation of the chest revealed crepitations. ECG on day 3 [Table/Fig-4] found to have normal sinus rhythm with no QRS abnormality and resolving early changes.
[DAY 3 ECG] - Controlled rate with resolution of earlier changes.
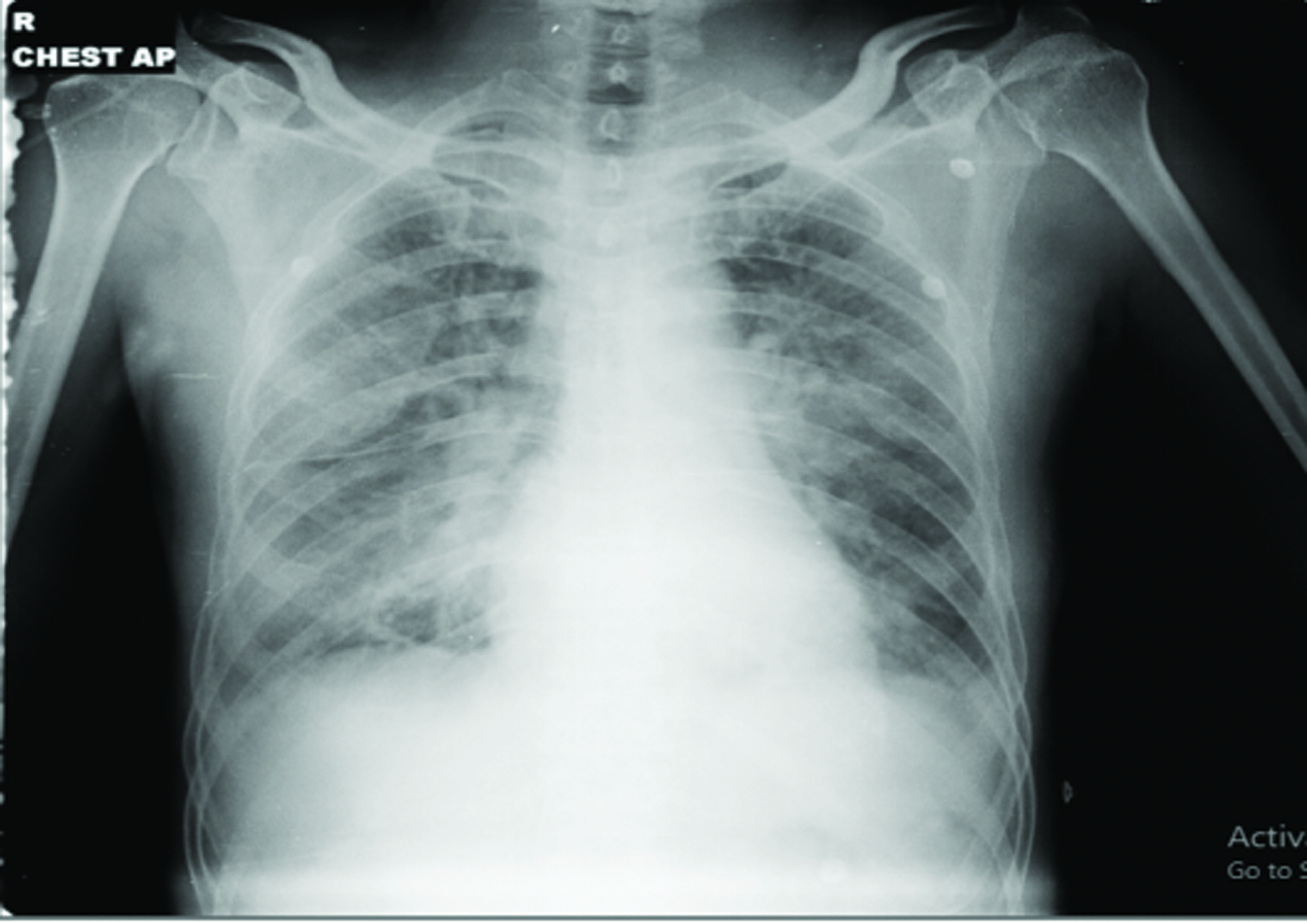
Chest X-ray Anteroposterior view [Table/Fig-5] showed acute pulmonary oedema and was started on NIV with low dose diuretics (Injection furosemide 10-20 mg intravenously). Then gradually NIV support was weaned off and he was kept on room air by the next day. On day 3, the patient showed gradual improvement in his clinical condition with normalising blood pressure (120/70 mm Hg). He was given antibiotics (injection ceftriaxone 1 gm i.v.), low dose diuretics (injection furosemide 20 mg i.v.), proton pump inhibitors (injection pantoprazole 40 mg i.v.) and other supportive medications. Chest physiotherapy and incentive spirometry were given periodically along with antibiotic coverage. Sputum culture showed Klebsiella pneumoniae-extended-spectrum β-lactamases (ESBL) producer. Blood and urine cultures found to be sterile. Antibiotics continued according to sensitivity pattern. The patient was eventually discharged after seven days of hospital stay with normal haemodynamic condition.
[Day 3 x-ray chest anteroposterior view]- features of acute pulmonary oedema.
Discussion
Minoxidil is a powerful direct acting vasodilator that was used clinically as an oral antihypertensive drug in combination with beta blockers and diuretics in cases of hypertension refractory to other antihypertensives [1]. Exact mechanism of action of Minoxidil is not clear. Minoxidil is used topically to treat androgenic alopecia of the scalp (2%, 5% minoxidil solutions) due to its hair growth stimulatory effect [2]. Minoxidil, when taken orally, may result in severe hypotension by direct arteriolar vasodilation, reflex tachycardia or reflux increase in cardiac output and myocardial contractility mediated by the sympathetic nervous system and it results in dynamic ECG changes, acute coronary syndrome, acute kidney injury [3-5]. Immediate resuscitation of the patient is mandatory to improve the haemodynamic status and to reverse other dynamic changes that occurred.
Minoxidil, which was originally used for the treatment of hypertension, recently has been approved for the treatment of male pattern baldness [6]. Adverse effects of local application of minoxidil on scalp are rare and minor. Most commonly it can cause itching and irritation on the affected area with other dermatological complications and minor systemic effects due to its small resorption. The systemic administration of minoxidil is associated with more serious complications.
Minoxidil is activated in the liver and its action is to relax vascular smooth muscle by opening cell surface potassium channels causing an efflux of potassium, hyperpolarisation and relaxation of smooth muscle cells [7]. Minoxidil produces systemic hypotension by direct arteriolar vasodilatation [8]. It is associated with a reflex increase in cardiac output and myocardial contractility mediated by the sympathetic nervous system [9]. Maximal concentration in the blood is achieved 1 hour after oral administration, but due to delay of active metabolic formation, the maximal therapeutic effect appears much later [10].
At least 95% of orally administered minoxidil is absorbed from the gastrointestinal tract and is metabolised to the direct acting vasodilator minoxidil-N-O-sulphate and the less pharmacologically active minoxidil-O-glucuronide [11]. Minoxidil sulphate activates the Adenosine triphosphate (ATP)-modulated K+ channel. By opening K+ channels in smooth muscle and thereby permitting K+ efflux, it causes hyperpolarisation and relaxation of smooth muscle [12]. Like hydralazine, minoxidil dilates arterioles but not veins.
Maximal hypotensive effects may be delayed due to the delay in the formation of the active metabolite. Minoxidil has a plasma half life of 3-4 hour, but its duration of action is 24 hour or occasionally even longer. It has been proposed that the persistence of minoxidil in vascular smooth muscle is responsible for this discrepancy.
Petkosva L et al., showed that minoxidil if ingested orally lead to severe hypotension, acute coronary syndrome, compensatory tachycardia, subendocardial ischaemia and acute kidney failure. In the above study, first day ECG showed diffusely inverted T waves with depressed ST segments in v2-v6 and laboratory findings were leucocytosis, elevated CPK-NAC, CPK-MB and increased renal parameters. ECG changes completely resolved by third day and lab parameters were gradually returned to normal by tenth day [12]. Exactly similar clinical, ECG and lab findings have been noticed in the present case where ECG and lab abnormalities resolved in similar fashion.
The cardiac consequences of the baroreceptor-mediated activation of the sympathetic nervous system during minoxidil therapy are an increase in heart rate, myocardial contractility and myocardial O2 consumption [13]. Thus, myocardial ischaemia can be induced by minoxidil in patients with coronary artery disease [14]. Panchal SK et al., reported a case of minoxidil exposure (3000 mg). The features were severe hypotension, tachycardia, subendocardial ischaemia which were treated efficiently with combination of crystalloids and norepinephrine where patient responded immediately. Initial ECG changes reverted to normal within 1 day. The subendocardial ischaemia was believed to be caused by increased myocardial oxygen demand and decreased coronary perfusion pressure secondary to extreme hypotension and tachycardia and immediate initiation of norepinephrine instead of dopamine have minimised myocardial ischaemia [5]. Contrarily, in the present case, it took 3 days for complete resolution of ECG changes despite of dual inotrope usage.
Various cardiovascular manifestations were noted with different doses of minoxidil in earlier studies [9,12,15,16]. Low doses may produce hypotension and successive increase in doses results in tachycardia and myocardial ischaemia, which may be probably a compensatory mechanism for severe hypotension [7,16]. In this patient, there was tachycardia, severe hypotension and characteristic ECG changes similar to typical ECG changes mentioned in earlier studies [5,9,15]. The measurement of serum levels of minoxidil would strengthen our report, but minoxidil is not detectable in the routinely performed toxic screen analysis in our country [12].
Forrester MB studied 125 cases of minoxidil exposures that were reported to Texas Poison centres during 2000-2014, and found that most exposures involved were due to ingestion (92%), were unintentional (98%) and involved patients who were of age 1-2 years of age. This study showed most of patients were managed on site (62%) i.e., at home and with incidence of minimal adverse effects like vomiting [8].
Even though above study states that exposure to minoxidil causes mild symptoms perse in majority of children, adult population who were using it must be cautious and must keep minoxidil outreach to children and alcoholic addicts as few case reports showed severe cardiac manifestations due to minoxidil exposure [4,17].
Conclusion(s)
Accidental ingestion of topical minoxidil showed tachycardia, severe hypotension and characteristic ECG changes. Immediate resuscitation of a patient with fluid challenge and inotropes had a role in control of haemodynamic status over 2 days and to reverse other characteristic changes occurred in ECG occurred. Minoxidil, which is available for topical use, must be kept in a safe place by the user as it is equally dangerous for children and alcoholics, if ingested. The present case was one of clear cut example where it showed improper storage lead to accidental consumption of the substance in thought of it as an alcohol.
SGOT: Serum glutamic-oxalacetic transaminase; SGPT: Serum glutamic-pyruvic transaminase; ALT: Alanine aminotransferase; CRP: C-reactive protein test; ESR: Erythrocyte sedimentation rate;
PT/INR: Prothrombin time and international normalised ratio; APTT: Activated partial thromboplastin time; ABG: Arterial blood gases; CPK-NAC: Creatine phosphokinase-N-acetyl-cystein; CPK-MB: Creatine phosphokinase -MB; Troponin- I
[1]. Suchonwanit P, Thammarucha S, Leerunyakul K, Minoxidil and its use in hair disorders: A reviewDrug Des Devel Ther 2019 13:2777-86.10.2147/DDDT.S21490731496654 [Google Scholar] [CrossRef] [PubMed]
[2]. Sica DA, Minoxidil: An underused vasodilator for resistant or severe hypertensionJ Clin Hypertens Greenwich Conn 2004 6(5):283-87.10.1111/j.1524-6175.2004.03585.x15133413 [Google Scholar] [CrossRef] [PubMed]
[3]. Mundt HM, Matenaer M, Lammert A, Göttmann U, Krämer BK, Birck R, Minoxidil for treatment of resistant hypertension in chronic kidney disease-A retrospective cohort analysisJ Clin Hypertens Greenwich Conn 2016 18(11):1162-67.10.1111/jch.1284727246772 [Google Scholar] [CrossRef] [PubMed]
[4]. Claudet I, Cortey C, Honorat R, Franchitto N, Minoxidil topical solution: An unsafe product for childrenPediatr Emerg Care 2015 31(1):44-46.10.1097/PEC.000000000000030125426682 [Google Scholar] [CrossRef] [PubMed]
[5]. Panchal SK, Mudgalkar N, Reddy KR, Minoxidil poisoning presenting as acute coronary syndrome: A rare case scenarioInt J Res Med Sci 2017 2(2):784-85.10.5455/2320-6012.ijrms20140583 [Google Scholar] [CrossRef]
[6]. Goren A, Naccarato T, Minoxidil in the treatment of androgenetic alopeciaDermatol Ther 2018 31(5):e1268610.1111/dth.1268630155952 [Google Scholar] [CrossRef] [PubMed]
[7]. Kikuchi S, Fujita Y, Onodera M, Fujino Y, Inoue Y, Prolonged hypotension induced by ingesting a topical minoxidil solution: analysis of minoxidil and its metabolitesAcute Med Surg 2016 3(4):384-87.10.1002/ams2.19629123818 [Google Scholar] [CrossRef] [PubMed]
[8]. Forrester MB, Pediatric minoxidil exposures reported to Texas poison centresPediatr Emerg Care 2018 34(6):413-16.10.1097/PEC.000000000000122629112111 [Google Scholar] [CrossRef] [PubMed]
[9]. Gheshlaghi F, Zoofaghari S, Dorooshi G, Unstable angina: A rare presentation of minoxidil intoxication: A case report and literature reviewJ Res Pharm Pract 2018 7(4):210-12.10.4103/jrpp.JRPP_18_2330622990 [Google Scholar] [CrossRef] [PubMed]
[10]. Song Y, Chin ZW, Ellis D, Lwin EMP, Turner S, Williams D, Stability of an extemporaneously compounded minoxidil oral suspensionAm J Health Syst Pharm 2018 75(5):309-15.10.2146/ajhp16045729472513 [Google Scholar] [CrossRef] [PubMed]
[11]. Gollasch M, Welsh DG, Schubert R, Perivascular adipose tissue and the dynamic regulation of Kv7 and Kir channels: Implications for resistant hypertensionMicrocirculation 2018 25(1):e1243410.1111/micc.1243429211322 [Google Scholar] [CrossRef] [PubMed]
[12]. Petkovska L, Petronievik Z, Chibishev A, Petkovski D, Stevcevska A, Minoxidil overdosage: A case reportMakedon Med Pregl 2016 70(2):104-07.10.1515/mmr-2016-0020 [Google Scholar] [CrossRef]
[13]. Pasala KK, Gujja K, Prabhu H, Vasavada B, Konka S, Short-term minoxidil use associated with pericardial effusion and cardiac tamponade: An uncommon presentationAm J Ther 2012 19(6):e186-88.10.1097/MJT.0b013e3181f5371c21519225 [Google Scholar] [CrossRef] [PubMed]
[14]. Brondfield M, Wu L, Benowitz N, Minoxidil-Associated Pleuropericardial EffusionJ Gen Intern Med 2016 31(9):110510.1007/s11606-016-3624-326951281 [Google Scholar] [CrossRef] [PubMed]
[15]. Oye M, Oye M, Ali A, Signs of early cardiac tamponade induced by MinoxidilAm J Emerg Med 2021 40:226.e1-e2.10.1016/j.ajem.2020.07.05032778436 [Google Scholar] [CrossRef] [PubMed]
[16]. Shashikala TP, Singh R, Muthukrishnan J, Refractory shock following ingestion of topical minoxidil solutionMed J Armed Forces India 2016 72(Suppl 1):S133-34.10.1016/j.mjafi.2016.02.00728050093 [Google Scholar] [CrossRef] [PubMed]
[17]. Aprahamian A, Escoda S, Patteau G, Merckx A, Chéron G, Minoxidil intoxication, the pharmacological agent of a hair lotionArch Pediatr 2011 18(12):1302-04.10.1016/j.arcped.2011.08.03122001642 [Google Scholar] [CrossRef] [PubMed]