Ovarian cancer is one of the most lethal cancers of the female reproductive system. Surface epithelial tumours form two thirds of all ovarian neoplasms and 90% of all ovarian cancers are surface Epithelial Ovarian Carcinomas (EOCs) [1]. Despite advanced treatment protocol, approximately 47% of women survive five years after diagnosis, and among women diagnosed with late-stage disease, five-year survival is only 29% [2]. High fatality of this neoplasm is due to lack of effective screening methods and the absence of early and specific signs and symptoms in the early stages of tumour [3]. So, identification of prognostic factors to predict the risk for metastasis, recurrence and patient survival will be the major development in the management of ovarian carcinoma.
Angiogenesis is the physiological process of new blood vessel formation, also a fundamental step in the transition of tumours from a dormant state to a malignant state. Without angiogenesis, tumour expansion cannot proceed beyond 1 to 2 mm because tumour proliferation is dependent on oxygen and nutrient supply to the cells and waste removal from, the tumour into the surrounding medium. The newly formed, immature, and leaky capillaries help the process of metastasis due to their fenestrated basement membranes allowing greater accessibility to tumour cells [4].
Because of the importance of angiogenesis in ovarian neoplasms, identification of growth factor related to ovarian angiogenesis is necessary in terms of diagnosis and therapy, therefore patient survival. The VEGF is a multifunctional cytokine which stimulates angiogenesis and increases microvascular permeability by binding to specific receptors on vascular endothelial cells. It also promotes the proliferation, survival, and migration of endothelial cells and is essential for blood vessel formation [5]. Increased expression of VEGF in ovarian carcinomas shows disease progression and poor prognosis and has been suggested as an independent prognostic factor for overall survival by multivariate analysis of survival [6].
So, with recent molecular studies, the development of novel VEGF targeting agents (anti-VEGF therapies) is regularly used for the treatment of ovarian cancer [7]. The aim of present study was to see the expression of VEGF in the spectrum of various types of epithelial ovarian neoplasms by IHC and to examine its relationship with histological types, grading, staging and tumour metastasis in malignant cases.
Materials and Methods
This was an observational cross-sectional study conducted on patients with ovarian neoplasms from August 2018 to January 2020 in Department of Pathology in collaboration with Department of Obstetrics and Gynaecology, SCB Medical College, Cuttack, India. Ethical Committee approval was obtained from Institutonal Ethics Committee of S.C.B. Medical College (Reference no. IEC/IRB No.819/11.3.19).
Inclusion criteria: Patients admitted to Department of Obstetrics and Gynaecology of SCB Medical College during the study period who underwent surgery, with histopathological diagnosis of epithelial ovarian neoplasms (both benign and malignant) and signed informed consent were included in the study.
Exclusion criteria: Patients with non epithelial ovarian neoplasms, inflammatory conditions of ovary, and post chemotherapy ovarian cancer were excluded from study.
Study Procedure
The present study included 50 consecutive cases of epithelial ovarian neoplasms. Clinical details, family history, past history, investigation findings were collected in each case. Surgical specimens of all ovarian masses fixed in 10% buffered neutral formalin for 18 to 24 hours were then embedded in paraffin wax, cut into 3 μm thick sections and stained with routine Hematoxylin and Eosin (H&E) stain. Histopathology sections were evaluated and categorised into different subtypes of surface epithelial neoplasms, benign/malignant and if malignant into various grades. The IHC was carried out manually by using a ready to use mouse monoclonal VEGF antibody by PathnSitu company on blocks rich in tumour tissue. Antigen retrieval was performed in conventional microwave method at 640 watts for two cycles and 480 watts for one cycle, five minutes each with antigen retrieval solution (Tris Buffer). Neutralisation of tissue endogenous peroxidase enzyme was done using peroxidase block, followed by primary antibody and secondary antibody as per conventional method. On examination of slides, positive staining for VEGF was observed as granular, brown cytoplasmic staining in the tumour cells. The expression of VEGF was scored by two individual pathologists.
The scoring was done by measuring two parameters: the intensity of immunoreaction and quantity of VEGF positive cells. The value scores for intensity of cytoplasmic staining were classified as negative, weak, medium and strong (0 for absent or negative, +1 for mild or weak, +2 for moderate or medium, +3 for severe or strong). The quantitative method used percentage of positive tumour cells (0 for absent staining, 1 for 1 to 10% staining, 2 for 11 to 50% staining, 3 for 51% to 100% staining). After combining the two scores (or the semi-quantitative method) to get the final scores, the surface epithelial tumours were categorised as high VEGF expressors (Scores 5 and 6) and low VEGF expressors (Scores 4 and below) [3].
Statistical Analysis
Data analysis was done using appropriate statistical method by SPSS software version 21.0 and statistical significance was tested by using Pearson’s chi-square test. The p<0.05 was considered significant.
Results
Total 50 cases of ovarian surface epithelial ovarian neoplasms were studied. The age of the patients ranged from 15 to 75 years, with highest number of cases in 4th and 5th decades (27 cases=54%) and a median age of 45 years [Table/Fig-1]. The most common histologic type was serous (28 cases=56%), followed by mucinous (18 cases=36%), endometrioid (two cases=4%), one case each of Brenner (2%) and clear cell type (2%) [Table/Fig-2]. Of the 50 cases, there were 19 (38%) benign neoplasms, six (12%) tumours of borderline malignant potential and 25 (50%) malignant neoplasms [Table/Fig-2]. Mean age of benign, borderline, and malignant cases were 36.94, 42, 51.28 years, respectively. In the age group above 40 years, 22 out of 34 cases (64.7%) were malignant tumours but below 40 years only three out of 16 cases (18.75%) were malignant. The p-value was less than 0.001 which was statistically significant. So, incidence of malignant neoplasms was significantly associated with increased age p<0.001.
Most common clinical presentation was pain abdomen (23 cases=46%) followed by lump in abdomen in 19 cases (38%), gastrointestinal problems like dyspepsia, bloating, abdominal fullness (10 cases=20%), abnormal bleeding per vaginum (6 cases=12%) and urinary problems (2 cases=4%). Family history of breast or ovarian carcinoma was observed in 5 cases (10%), all of which were observed in malignant cases. In this study 15 out of 50 cases (30%) presented with ascites, among which 20% (19 cases) were malignant tumours and rest 10% (5 cases) comprised of benign and borderline tumours. Ascitic fluid positive for malignant cells was present in 4 patients (8%) with malignant tumours rest came out to be reactive effusion. Omentum and lymph nodes were removed with all malignant ovarian tumours. Out of the patients in advanced stage (Stage III and IV) 56% (28 cases) revealed either retroperitoneal lymph node or omental metastasis.
On gross examination, 32 (15%) cases were of mixed solid and cystic consistency, 56% (28) cases were purely cystic and 28% (14) were purely solid. Among 50 cases, 26% (13) cases were bilateral, all of which were malignant neoplasms. The rest 37 cases (74%) had unilateral tumours, comprising of 34% (17) right sided and 40% (29) left sided tumours.
On histologic grading of 25 malignant tumours, 17 (68%) were high grade, constituting 12 (48%) cases of serous cystadenocarcinoma, four mucinous cystadenocarcinoma and one clear cell carcinoma. Rest, including two endometrioid carcinomas were of low histologic grade [Table/Fig-2]. The International Federation of Gynaecology and Obstetrics (FIGO) staging of patients showed 16 patients (64%) in advance stage (stage III-48%, stage IV-16%) and 9 patients (36%) in early stage (stage I-12%, stage II- 24%).
Age distribution of surface epithelial tumours according to biological behaviour.
| Age (years) | Benign | Borderline | Malignant | Total (%) |
|---|
| 0-19 | 2 | 0 | 0 | 2 (4) |
| 20-39 | 9 | 2 | 3 | 14 (28) |
| 40-59 | 6 | 4 | 17 | 27 (54) |
| ≥60 | 2 | 0 | 5 | 7 (14) |
| Total | 19 | 6 | 25 | 50 (100) |
Distribution of VEGF according to biological behaviour.
| Histological type | Histological subtype | Absent expression | Low expression | High expression | Total |
|---|
| Benign | Serous cystadenoma | 1 | 9 | 0 | 10 (20%) |
| Mucinous cystadenoma | 5 | 3 | 0 | 8 (16%) |
| Benign brenner | 0 | 1 | 0 | 1 (2%) |
| Total | 6 (12%) | 13 (26%) | 0 | 19 (38%) |
| Borderline | Borderline serous tumour | 0 | 1 | 1 | 2 (4%) |
| Borderline mucinous tumour | 1 | 2 | 0 | 3 (6%) |
| Serous cyst adenofibroma of borderline malignant potential | 0 | 1 | 0 | 1 (2%) |
| Total | 1 (2%) | 4 (8%) | 1 (2%) | 6 (12%) |
| Malignant | Serous cystadeno-carcinoma low grade | 0 | 1 | 2 | 3 (6%) |
| Serous cystadeno-carcinoma high grade | 0 | 2 | 10 | 12 (24%) |
| Mucinous cystadeno-carcinoma low grade | 0 | 2 | 1 | 3 (6%) |
| Mucinous cystadeno-carcinoma high grade | 0 | 0 | 4 | 4 (8%) |
| Endometrioid carcinoma | 0 | 2 | 0 | 2 (4%) |
| Clear cell carcinoma | 1 | 0 | 0 | 1 (2%) |
| Total | 1 (2%) | 7 (14%) | 17 (34%) | 25 (50%) |
| Total | 8 (16%) | 24 (48%) | 18 (36%) | 50 (100%) |
All cases were subjected to VEGF IHC and 42 of the 50 cases (84%) of surface epithelial ovarian neoplasms were VEGF positive. A 36% (18 out of 50) showed high expression and 64% (32 from 50) cases showed low or no expression of VEGF. The VEGF expression was absent in 8 (16%) tumours comprising of five mucinous cystadenoma, one serous cystadenoma and one case of borderline mucinous tumour. Out of the 19 benign epithelial neoplasms, 13/19 (68.4%) showed low expression and 6 (31.5%) had no expression of VEGF. High expression of VEGF was not observed in any of the cases in the benign category. Among the six cases of borderline malignant potential, majority (4=66.67%) cases showed low VEGF expression, one case each revealed high and no expression. Likewise, out of malignant tumours, maximum (17/25=68%) showed high expression of VEGF and only one case didn’t show any VEGF staining [Table/Fig-2]. The VEGF expression was significantly higher in carcinomas as compared to benign and borderline neoplasms (p≤0.001).
Comparative analysis of VEGF expression among different histologic types is shown in [Table/Fig-3]. Expression is maximum (27/28 or 96%) in serous category [Table/Fig-3] in comparison to 12/18 in mucinous category (66.7%, [Table/Fig-4]) and only low expression in Brenner and clear cell types. Both cases of endometrioid carcinoma were found to be low expressors of VEGF [Table/Fig-5]. In the benign category, out of the 10 serous tumours, 9 (90%) showed low expression and 1 (10%) no expression which was high in comparison to other categories. Among the borderline categories, serous and mucinous categories were of equal number but the expression of VEGF was mostly low or absent comprising 83.3% (5 out of 6); only one case of borderline serous tumour showed high expression of VEGF. Serous morphology overwhelmingly expressed VEGF throughout the spectrum of benign, borderline and malignant neoplasms. Twelve (81.8%) out of 15 cases of serous carcinomas showed high VEGF expression [Table/Fig-2]. All malignant surface epithelial tumours had VEGF expression either low (28%) or high (68%) except the single clear cell carcinoma [Table/Fig-6] in present study.
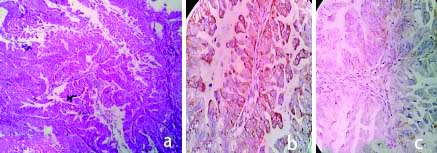
a) H&E,100X, Serous Adenocarcinoma; b) IHC with VEGF, Serous Adenocarcinoma (High expression:Score-6) 400X.
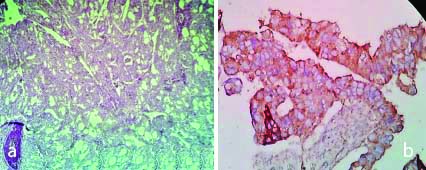
a) H&E,100X, Mucinous adenocarcinoma; b) IHC with VEGF, Mucinous Adenocarcinoma (High expression: Score-5) 400X; c) IHC with VEGF, Mucinous Adenocarcinoma (Low expression: Score-2) 400X.
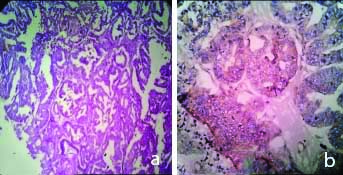
a) H&E, 100X, Endometrioid Carcinoma; b) IHC with VEGF, Endometrioid Carcinoma (Low expression: Score-3) 400X.
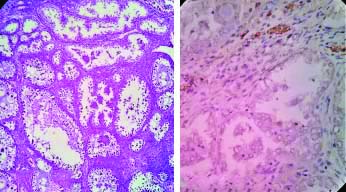
a) H&E, 100X, Clear Cell Carcinoma; b) IHC with VEGF, Clear cell Carcinoma (Absent expression: Score-0) 400X.
Histologic grade and VEGF expression-14/17(82.3%) of the high-grade neoplasms showed high expression of VEGF [Table/Fig-7]. Only one case of clear cell carcinoma being high in grade showed absent expression. A significant association (p=0.024) was seen between grade of carcinomas and VEGF expression.
VEGF expression with grade, stage and metastasis of ovarian epithelial carcinoma.
| Variables | Grade/Stage | High expressors | Low expressors | Total |
|---|
| Grade | High grade | 14 (56%) | 3 (12%) | 17 (68%) |
| Low grade | 3 (12%) | 5 (20%) | 8 (32%) |
| Stage | Low Stage (I and II) | 3 (12%) | 6 (24%) | 9 (36%) |
| Advanced stage (III and IV) | 14 (56%) | 2 (8%) | 16 (64%) |
| Metastasis | (Omentum/ lymph node) | 10 (40%) | 2 (8%) | 12 (48%) |
Stage and VEGF expression- 14 out of 16 patients (87.5%) who presented in advanced stage (stage III and IV) were high expressors and 6 out of 9 patients (66.67%) presented in early stage (stage I & II) were low expressors [Table/Fig-7]. High VEGF expression was significantly associated with advanced stage of disease in the present study (p=0.05).
Tumour metastasis and VEGF expression- Out of 14 patients in advanced stage, 12 showed either omental and/or retroperitoneal lymph node metastasis. The VEGF IHC revealed 10 to be high expressors (83.3%) and only 2 low expressors (16.7%) with significant p-value (<0.05).
Discussion
Epithelial ovarian neoplasms are a heterogeneous group of disease, comprising of several histotypes with distinct epidemiologic, molecular, and clinical features [8]. Despite recent studies on the origin and precursor lesions of ovarian cancer, advance treatment protocol could not provide optimum benefit to the patients of ovarian carcinomas; cure rates remain relatively unchanged with resultant high mortality. Tumour angiogenesis is one of the essential factors for tumour to grow and VEGF (a very potent angiogenetic factor), increases vaso-permeability, promote neovascularisation and causes tumour growth. It plays important role in tumour vascular endothelial cell proliferation and migration, as well as ascites generation. Suppressing the angiogenic pathways can facilitate the entry of immune effector cells and thereby reduce the presence of myeloid cells required for immune suppression. Till now, significant benefits have been obtained with antiangiogenic therapy as first-line therapy in fresh and recurrent ovarian cancers. VEGF inhibitor bevacizumab is now an established therapy but clinical data with immunomodulators are more limited however suggests that they could benefit some patients with recurrent/resistant disease [9]. Since ovarian neoplasms are dependent on angiogenesis, use of angiogenesis inhibitors are used as anticancer therapy. Immunohistochemical behaviour of VEGF correlates with angiogenic behaviour of tumour and prognosis. Hence, evaluation of VEGF in tumour cells can be used as key factor in deciding tumour response and therapy efficacy in ovarian cancers. So, authors studied expression of VEGF (by IHC) in 50 cases of surface epithelial ovarian neoplasms in two tertiary centres of Eastern India. Here, we discuss the data generated from the present study in the light of the research work conducted by previous workers in this field.
Out of the total 50 cases in the study, majority (25/50=50%) were malignant neoplasms followed by benign (38%) and borderline (12%) tumours. As samples have been taken from two different Institutions, one of which being Regional Cancer Institute, increased number of malignant tumours were noticed in present study. Janaki M et al., and Agarwal P et al., reported similar distribution [10,11]. Median age of patients was 45 years and mean age for malignant neoplasms was 51.28 years which correlates with findings of Premalata CS et al., [12]. Among surface epithelial tumours, serous category was the dominant type (56%) followed by mucinous (36%), endometrioid carcinoma (4%), Brenner (2%) and clear cell carcinoma (2%), unlike Agarwal P et al., where most common category was mucinous carcinoma and Premalata CS et al., where most common was serous carcinoma (64.1%), but followed by endometrioid carcinoma (12.8%) and mucinous carcinoma (9%) was less in incidence [11,12].
A 42 of the 50 cases (84%) of surface epithelial ovarian neoplasms showed VEGF expression. The expression was significantly higher in carcinomas as compared to benign and borderline neoplasms (p<0.001). This finding was comparable to studies of Inan S et al., and Garzetti GG et al., who also observed that VEGF immunostaining was higher in cases of cystadenocarcinomas than in borderline or benign neoplasms [13,14]. Out of the 25 malignant cases, 17 (68%) showed high VEGF expression. The VEGF immunostaining was found to be significantly higher in cystoadenocarcinomas, with the highest values detected in grade 3 neoplasms (FIGO) (p<0.001) [15]. Most of the high grade serous and mucinous carcinomas were high expressors of VEGF, two cases of endometrioid carcinoma showed low expression and one case of clear cell carcinoma showed no expression at all in present study.
Tumours of serous morphology overwhelmingly expressed VEGF throughout the spectrum of benign, borderline and malignant categories. About 12 (80%) out of 15 cases of serous carcinomas showed high VEGF expression. Brustmann H found focal or diffuse VEGF positivity in 51% of serous carcinoma [15].
While comparing tumour grade with expression of VEGF in this study, 82.3% of the high grade neoplasms were high expressors of VEGF indicating a significant association between grade of neoplasms and VEGF expression (p=0.024). Similar observation was found with Premalata CS et al., and Shen GH et al., having p-values 0.024 (present study), 0.0004 and 0.022, respectively [12,16]. In the study by Ranjbar R et al., well differentiated tumours (17.6%) showed positive and higher expression of VEGF compared to moderately (30.8%) and poorly (27.8%) differentiated tumours [17].
Most of the (10/12=83.3) stage III carcinomas and all (4/4 cases) stage IV carcinomas (with malignant pleural effusion) were high expressors of VEGF (14/16=87.5%). All of Stage I carcinomas (3 cases) and 3/6 cases of stage II carcinomas were low expressors. Thus, high VEGF expression was significantly associated with advanced stage of carcinoma (p=0.005). In the study done by Wang W et al., the expression rate of VEGF in ovarian cancer was higher than that in the normal group, and the later the tumour stage, the higher the positive expression rate of VEGF (p<0.001) [18]. Lozneanu L et al., observed that Endocrine Gland derived VEGF (EG-VEGF) is overexpressed mainly in high grade ovarian carcinomas (type II) than in low grade ones. Significant differences were also seen in EG-VEGF positive or negative expression and tumour stage and histological subtypes [19]. In the study by Brustmann H also, VEGF immunoreactivity was positively related with Topoisomerase II (TPII) alpha labeling indices (LI) (p=0.0055), adverse outcome (p=0.0052), high FIGO stage (p=0.0158), and high tumour grade (p=0.0303) [15].
The VEGF plays a crucial role in tumour metastasis. Initial studies revealed that VEGF driven angiogenesis is an early and important event in ovarian carcinogenesis [20,21]. Guo BQ and Lu WQ did a meta-analysis and found that the high/positive expression of VEGF as a prognostic biomarker in ovarian cancer [22]. Ovarian cancer cells overexpressing VEGF hold a metastatic advantage over those lacking VEGF. It contributes to intraperitoneal ovarian cancer dissemination via interacting with tumour microenvironment and malignant ascites [23,24]. In present study, omental and lymph node metastasis was observed in 12 cases (48%); out of which 10 cases (83%) were high expressors of VEGF. Sopo M et al., also observed that compared to primary high grade serous ovarian tumours, the related omental metastases showed higher expressions of VEGF-A (p = 0.022), VEGF-D (p = 0.010), and VEGF Receptor (VEGFR) (p = 0.046) [25].
The expression pattern of VEGF in tumour is important as antiangiogenetic drugs are increasingly being used successfully to treat colon, breast and lung carcinomas also some refractory and recurrent ovarian carcinomas [26-30]. In a review done by Arend RC et al., it is observed that combination of Poly Adenosine-diphosphate Ribose Polymerase (PARP) inhibitor with VEGF receptor inhibitor is giving a better result for advanced stage ovarian cancers than that of PARP alone [31]. The anti-VEGF therapy induces tumour hypoxia and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) expression, which recruits MDSCs (Myeloid-derived suppressor cells) and inhibits tumour immunity [32]. Targeting the GM-CSF can be the research thrust in management of resistant ovarian cancer in near future.
Limitation(s)
Paucity of cases and limited time period were few limitations of present study.
Conclusion(s)
As most epithelial ovarian cancers are dependent on VEGF for tumour progression, the findings of present study may be useful to select specific group of patients for targeted antiangiogenic therapy with agents against VEGF. Therapeutic agents targeting VEGF/VEGFR in clinical development for ovarian cancer are Bevacizumab (acts against all isoforms) and Aflibercept (against VEGF and VEGF-B). These can be administered in cases which are high expressors of VEGF with a good result.