The hyperpigmentary disorders, a common skin disorder affecting 10.8% of Indian population, characterised by darkening of the skin due to increased number or activity of melanocytes [1-3]. These disorders affect all skin types and any part of the body, yet, individuals with darker skin, including Asians, Blacks, Hispanics and American Indians are more susceptible. Though mostly asymptomatic, still it can have a great impact on the patient’s QOL with physical distress and psychological impact [2].
As there is no cure for hyperpigmentary disorders, therapy is non specific, empirical and symptomatic. Medications are often used alone or in combination, mainly aimed at inducing prolonged remission and suppressing the disease to a tolerable level. The management involves topical medications, systemic drug therapy and physical therapy. The topical medications include sunscreens, demelanising agents, immunomodulators like tacrolimus, retinoids and GCs [4-10]. Systemic therapy includes GCs and antioxidants [4,9,11]. Physical therapy includes chemical peels, microderma abrasion, laser & light therapies and mesotherapy [9-11].
Inspite of enormous studies recently, regarding the research related to biology of melanocytes, clinical aspects and advanced therapy, the outcome still remains unsatisfactory with respect to the treating doctor and the patient [1,9,12-14]. Because of the variability in the type, severity and duration of the disease and availability of wide therapeutic options, the prevalent pattern of treatment may vary in different geographical regions, ethnic groups and also in different medical establishments, depending upon the facilities and resources. Analysing such an outcome has a greater impact on the measurement of effects for clinical, therapeutic and health service research [14].
In spite of diverse range of therapeutic modalities, there is still a need to develop more potent and safe inhibitors of melanogenesis [15]. Vashi NA and Kundu RV, has categorised the appropriate treatment strategies for hyperpigmentation, in view of factors like lack of effective treatment, side-effects like postinflammatory hyperpigmentation with different treatment protocols, as well the cosmetic and emotional effect [13]. Khanna N and Rasool S has highlighted that there are currently no guidelines for the management of hyperpigmentation and it is hard to make effective comparisons between the outcomes due to variations in the nature of the disease and treatment modalities [8]. The impact of QOL, before and after treatment has not been addressed by many studies, except a pilot study, where there was an improvement in self-esteem after treatment [16].
As there is a paucity of studies regarding overall pattern of drug use and evaluating their effectiveness, safety, tolerability to various medications and QOL, hence the present study was taken up to generate useful and valid data. In the present study, the pattern of drug use, its tolerability and analysing the effect of prescribed medications with respect to QOL in hyperpigmentary disorders of skin was assessed prospectively in 102 participants in a tertiary care teaching hospital.
Materials and Methods
This prospective, observational study was conducted using purposive sampling among 102 newly diagnosed and untreated participants with hyperpigmentary disorders attending the Outpateint Department of Dermatology (OPD) of a Tertiary Care Hospital and Research Centre, Bangalore, India for a period of 18 months (from January 01, 2015 to June 30, 2016). Approval and clearance from the Institutional Ethics Committee (KIMS/IEC/2014/10/9) was obtained before beginning the study. Written informed consent was taken from all the study participants after fully explaining the study procedure to their satisfaction, in both English and Kannada language. Anonymity, confidentiality, and professional secrecy were maintained for all the study participants. Sample size was estimated using the formula 4 Pq/d2, {prevalence=10.8%, q=100-p, d (absolute precision)=5%} [1].
Inclusion criteria: Participants newly diagnosed as having hyperpigmentary disorder aged between 18-65 years, willing to give written informed consent were included in the study.
Exclusion criteria: Pregnancy, lactation, congenital pigmentary disorders, neoplastic disorders of skin, participants who were not ready for regular follow-up (every month) were excluded.
Procedure
Demographic and clinical data obtained from the newly diagnosed participants with hyperpigmentary disorders were recorded in a Case Record Form (CRF) and analysed to determine the pattern of drug utilisation-topical or systemic, therapeutic class of the drug, the drugs or drug combinations used, the type of formulation, method of administration/application, frequency and duration of use, tolerability of the medications, and the QOL.
Tolerability of the medications was assessed by monitoring any AEs. Health related (QOL) was assessed by 10-item (DLQI) [17]. There were three follow-up visits, thirty days apart for a period of three months to assess the appropriateness, changing trends in prescription pattern, tolerability and QOL. The prescribing physician was interviewed to ascertain the reasons for any change in treatment.
Statistical Analysis
The data collected was analysed by using descriptive statistics, namely mean, Standard Deviation (SD), frequency, percentage. Results on continuous measurements like age, DLQI scores were presented as mean±SD and results on categorical measurements were presented as number (%). Comparison of means of more than three groups was done using one-way analysis of variance (ANOVA) for DLQI. Significance was assessed at 5% level of significance. Multiple responses were reported in terms of percentages and total of such response were greater than sample size. The results were also depicted in the form of tables and graphs. Statistical software namely Statistical Package for the Social Sciences (SPSS) version 20 was used for the analysis of data and Microsoft Word and Excel to generate graphs and tables.
Results
Altogether 102 participants were enrolled for the study. The mean age was 33.71±10.68 years in males and 34.07±10.27 years in females. Majority (83.33%) of the participants were in the age group of 18-40 years. There were (30.40%) male participants and (69.60%) female participants. Melasma was the most commonly observed disorder in (29.4%) participants, closely followed by Post Inflammatory Hyperpigmentation (PIH) in (28.4%) participants [Table/Fig-1].
Clinical diagnosis-types of hyperpigmentary disorders.
| Diagnosis |
|---|
| Hyperpigmented disorder | No. of subjects n (%) |
|---|
| Acanthosis nigricans | 7 (6.86) |
| Melasma | 30 (29.41) |
| Postinflammatory hyperpigmentation | 29 (28.43) |
| Photomelanosis | 9 (8.82) |
| Frictional melanosis | 3 (2.94) |
| Macular amyloidosis | 4 (3.94) |
| Periorbital Melanosis (POM) | 6 (5.88) |
| Freckles | 3 (2.94) |
| Seborrhoeic melanosis | 2 (1.96) |
| Vitamin B12 deficiency | 2 (1.96) |
| Drug induced hyperpigmentation* | 1 (0.98) |
| Lichen Planus Pigmentosus (LPP) | 2 (1.96) |
| Hypertrophic lichen planus | 4 (3.92) |
| Total | 102 (100) |
*On Multidrug therapy for Multibacillary type of leprosy (MDT-MB)
Treatment modalities
Among the various treatment modalities used in hyperpigmentary disorders, topical administration was the most common mode of therapy in (37.25%) participants and it included sunscreens, demelanising agents, GCs, calcineurin inhibitors, keratolytics, polysiloxanes, emollients and antibiotics like clindamycin 1% gel. Topical along with systemic agents were administered in (62.7%) participants which included {topical (sunscreens, demelanising agents, GCs, calcineurin inhibitors, keratolytics, polysiloxanes, emollients) and various adjuvants used as systemic agents (GCs, antifibrinolytics, antihistamines, vitamins, antioxidants, antimalarial, and retinoids)}.
Class of drugs
Among the various classes of drugs used in 102 participants, demelanising agents and combinations were the most common class of drugs prescribed in 91 (89.21%) participants.
In (43.13%) participants sunscreen agents with SPF26 or more (UVA+UVB) were advised alone or with demelanising agents and the next common class of drugs prescribed in (29.41%) participants, were antifibrinolytics like tranexamic acid 250 mg, twice daily, which was used as a definitive therapy to reduce pigmentation. Other drugs were prescribed as adjuvants in 70 (68.6%) participants, which included topical emollients, clindamycin gel, oral antioxidants and antihistamines were used to provide symptomatic relief [Table/Fig-2].
Class of drugs used in Hyperpigmentary disorders.
| Class of drugs | N (%) |
|---|
| Demelanising agents (hydroquinone 4%) and combinations | 91 (89.21) |
| Antifibrinolytics (tranexamic acid 250 mg) | 30 (29.41) |
| Glucocorticoids and combinations | 24 (23.52) |
| Calcineurin inhibitors (tacrolimus 0.1%) | 13 (12.74) |
| Keratolytics | 9 (8.82) |
| Sunscreens | 44 (43.13) |
| Vitamins (mecobalamin+ pyridoxine+ folic acid) | 2 (1.96) |
| Retinoids (isotretinoin 20 mg) | 3 (2.94) |
| Antimalarial (hydroxychloroquine 200 mg) | 1 (0.98) |
| Polysiloxanes (peptide+ceramide) | 2 (1.96) |
| Others (emollients, antioxidants, antihistamines) | 70 (68.6) |
Demelanising agents and combinations
Of the demelanising agents and combinations, one or more drugs of same class or same drugs of different formulation were used in same/one participant. Out of the 91 (89.21%) participants receiving demelanising therapy, topical HQ was the most commonly prescribed monotherapy in (30.76%) participants followed by triple formula which is a combination of topical demelanising agent+retinoids (tretinoin)+steroids (mometasone, fluocinolone). In 20.87% participants, combination of {(demelanising agent (HQ and kojic acid (KA)+keratolytics (Glycolic Acid (GA)+antioxidants like vitamin E (soap or cream) as an adjuvant demelanising agent)} demonstrates improvement in epidermal melasma, while combined use of sunscreens along with demelanising agents acts as photoprotective measure and prevent further repigmentation. Newer botanical extracts (arbutin, liquorice, mulberry, galanga ester, pterowhite) were used alone or as adjuvants to HQ based therapies in (19.78%) participants [Table/Fig-3].
Demelanising agents and combinations (n=91, 89.21)*.
| Drug and concentration† | N (%) |
|---|
| Demelanising agent alone (n=28,30.76%) |
| Hydroquinone 4% | 28 (30.76) |
| Botanicals (n=18,19.78) |
| Kojic acid+arbutin+mulberry+liquorice+vitamin E+tetrahydrocurcumin | 8 (8.79) |
| Liquorice+galanga ester+pterowhite | 8 (8.79) |
| Niacinamide+lecithin based combination | 2 (2.19) |
| Demelanising+Sunscreen (n=13,14.28%) |
| Hydroquinone 4%+oxybenzone | 10 (9.8) |
| Kojic acid 2%+arbutin 1.5%+octinoxate 7.5%+allantoin 0.4% | 3 (3.29) |
| Demelanising+Keratolytic+Antioxidant (n=19, 20.87%) |
| Hydroquinone 1%+kojic acid 2%+GA 2%+vitamin E (soap and cream) | 18 (19.78) |
| Hydroquinone 2%+kojic acid 2%+aluminium silicate 1%+lactic acid 0.2%+vitamin C 0.5% | 1 (1.09) |
| Demelanising+Sunscreen+Keratolytic+Antioxidant (n=1, 1.09%) |
| Kojic acid dipalmitate 2.5%+arbutin 1.5%+mulberry1%+octinoxate 7.5%+GA 3%+vitamin E 1%+grapeseed oil 2%+allantoin 0.4% | 1 (1.09) |
| Demelanising+Sunscreen+Antioxidant (n=2, 2.19%) |
| Kojic acid dipalmitate 2%+arbutin 1.5%+mulberry 1.0%+octinoxate 7.5%+vitamin E 1.0% | 2 (2.19) |
| Demelanising+Retinoid+Steroid (n=22, 24.17%) |
| Hydroquinone 2%+mometasone 0.1%+tretinoin 0.025% | 20 (21.97) |
| Hydroquinone 2%+fluocinolone 0.01%+tretinoin 0.05% | 2 (2.19) |
*n=Total number of subjects receiving the particular class of drug;
N=Number of subjects receiving the individual drug. One or more drugs of same class or same drugs of different formulation were used in same/one subject;
†Concentration is expressed as percentage, based on w/w (weight/weight)
Keratolytics
An 8.82% participants received keratolytics and combinations, out of which the most frequently prescribed Fixed Dose Combination (FDC) in (6.82%) participants was topical (Glycolic acid 1% (GA)+salicylic acid 2%) either as face wash, cream or chemical peels in PIH, sebomelanosis.
Glucocorticoids and combinations
In terms of GCs and combinations (n=24,23.52%), topical GCs alone (n=18, 17.64%) that includes clobetasol (n=6, 5.88%), mometasone (n=6, 5.88%), desonide (n=5, 4.90%), betamethasone (n=1, 0.98%) or along with keratolytic (clobetasol+salicylic acid n=2,1.96%) was more commonly used than systemic GCs (n=4, 3.92%) like triamcinolone (n=1, 0.96%), prednisolone (n=3, 2.94%).
Number of drugs per prescription
A total of 80.39% of the participants required three or more drugs either as combined therapy or as FDCs, more than one drug from the same therapeutic class or same drug as different formulation, with a mean of 3.01±1.01.
Quality Of Life (QOL)
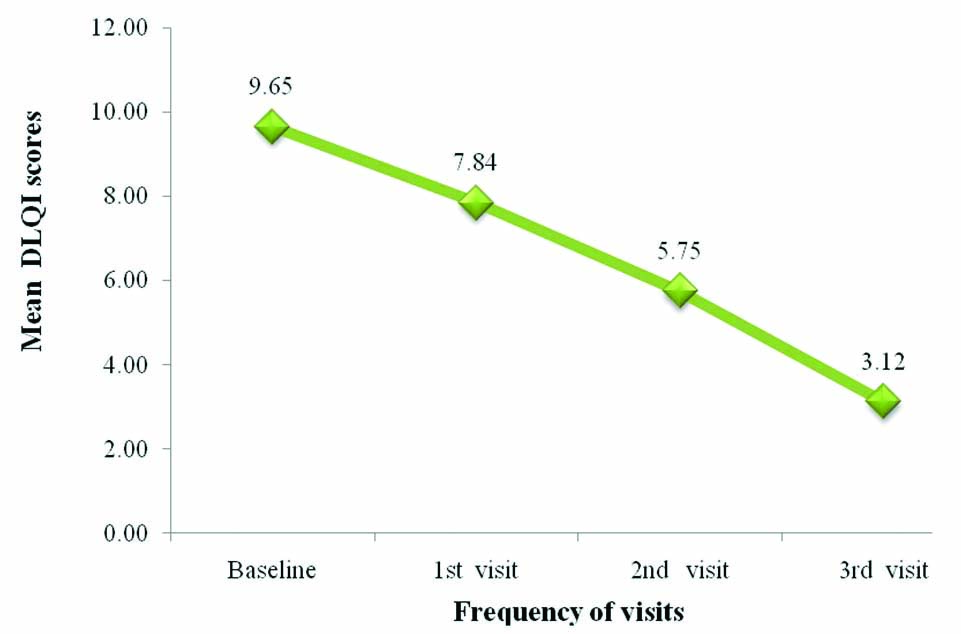
At the baseline visit, all participants had DLQI scores ranging from 2-5, that indicates small effect on QOL, at the third visit, only 60% of the participants had scores ranging from 2-5, 40% had scores ranging from 0-1=no effect on QOL. Overall, the minimum score of QOL was zero and maximum score was 18. The mean score was 9.67±5.313 during baseline and 3.12±2.40 during 3rd visit.
DLQI scores from baseline to the end of the study (three months) and baseline to each visit, was significant with p-value <0.01, suggesting improvement in QOL following treatment [Table/Fig-4].
Dermatology Life Quality Index (DLQI) overall score (p-value <0.01)
Reasons for Change in treatment
Among 8 (7.84%) participants, the most common reason for change in treatment was inadequate clinical response. Of which, participants with PIH (n=3, 37.5%), photomelanosis (n=2, 25%), melasma (n=1, 12.5%) who were on topical demelanising agents (HQ based triple formula/HQ alone/botanical extracts) were substituted with chemical peels. A 25% participants with melasma, who were on HQ or KA alone, were substituted with fluocinolone based triple combination HQ+GA+KA+vitamin E based combination.
Adverse events
Adverse Events (AEs) like erythema (n=19, 18.62%), pruritis (n=18, 17.64%), irritation (n=14, 13.72%), skin dryness (n=11, 10.78%), were most commonly encountered during the first visit (one month) probably due to HQ, mometasone+HQ+tretinoin combination. Dryness of skin was reported with systemic isotretinoin which was self-limiting and disappeared during subsequent visits and in few Participants symptomatic relief was provided.
The AEs like acneiform eruption (n=5, 4.90%), rosacea (n=3, 2.94%) were commonly seen in participants who were on long term topical GCs therapy on the second visit. Allergic Contact Dermatitis (ACD) was reported among 4 (3.92%) participants receiving HQ therapy during first visit (1 month) [Table/Fig-5].
Adverse Events (AEs) at each visit.
| Adverse events | Baseline | 1st visit (30 days) | 2nd visit (60 days) | 3rd visit (90 days) |
|---|
| Pruritis | 0 | 18 | 4 | 0 |
| Irritation | 0 | 14 | 4 | 0 |
| Erythema | 0 | 19 | 4 | 1 |
| Burning sensation | 0 | 8 | 3 | 0 |
| Skin dryness | 0 | 11 | 8 | 0 |
| Dry lips | 0 | 7 | 4 | 0 |
| Rash | 0 | 5 | 2 | 0 |
| Wrinkles | 0 | 0 | 1 | 0 |
| Telangiectasia | 0 | 0 | 1 | 0 |
| Hypertrichosis | 0 | 0 | 2 | 0 |
| Acneiform eruption | 0 | 3 | 5 | 1 |
| Steroid induced rosacea | 0 | 0 | 3 | 0 |
| Hypopigmentation | 0 | 0 | 2 | 0 |
| Allergic contact dermatitis | 0 | 4 | 1 | 0 |
| Total* | 0 | 89 | 44 | 2 |
*One or more side-effects were seen in same subject
Treatment was changed due to AEs in 6 (5.88%) participants, of which 4 (66.6%) participants who were on either HQ or HQ-based triple combination therapy (HQ+tretinoin+mometasone), complained of irritation, pruritis, erythema and burning. Subsequently HQ was substituted with KA, and other symptomatic (moisturisers) measures were administered. HQ-induced ACD in one (16.6%) participant, so it was stopped and desonide was given.
One (16.6%) participant on MDT-MB therapy complained of hyperpigmentation (could be clofazimine induced), it was stopped and ROM (Rifampicin 600 mg+Ofloxacin 400 mg+Minocycline 100 mg) therapy was initiated under supervision for 12 months.
Discussion
In the present prospective observational study, the pattern of drug use in hyperpigmentary disorders, its tolerability and the effect of prescribed medications with respect to QOL was assessed in 102 participants attending dermatology OPD of KIMS Hospital and Research Centre, Bangalore. It was found that the most common class of drug prescribed was demelanising agent and its adverse effect was erythema, there was significant improvement in QOL after treatment.
Out of the 102 study participants, majority were in the age group between 18 to 40 years and the number of females was more than males. The most common disorder in the present study was melasma (29.4%), akin to studies [1,3,18].
In the present study, the most common drugs prescribed were demelanising agents, sunscreens and antifibrinolytics. Topical therapy was the basis in the management, of which the most commonly used demelanising agents in this study included 4% HQ, triple formula (HQ 2%+mometasone 0.1%+tretinoin 0.025%), newer regimens like (HQ 1%+GA 2%+KA 2%+vitamin E) and newer botanical extracts- either as monotherapy or adjuvants to HQ similar to other studies [4,8-12,16,19-25]. Topical therapy was substituted with tranexamic acid 250 mg in melasma, PIH and photomelanosis [9-11,16]. In this study, sunscreens (avobenzone 2%+octylmethoxycinnamate 7.5%+oxybenzone 3%+zinc oxide 8% gel) were used as photoprotective agents, similar to other studies [9-11]. Tretinoin and KA were also used which was consistent with few studies. [16,26-31]. [Table/Fig-6] depicts a summary of related studies [4,9,21,23-25,27,30]. As per a study by Desai SR, zinc sulfate, arbutin, azelaic acid, KA, cosmeceuticals can be added as a second topical, or as monotherapy. Chemical peels (salicylic acid, GA) in combination with topical therapy may be used as second line therapy [12].
Summary of common drugs used and its adverse effects in hyperpigmentary disorders of skin [4,9,21,23-25,27,30].
| Authors | Significant details | Common drugs | Adverse effects |
|---|
| Chan R et al., [21] | 120 East and South-East Asian patients, Melasma | Triple combination {Fluocinolone acetonide 0.01%, hydroquinone (HQ) 4%, tretinoin 0.05%}, HQ 4% | Skin irritation, dryness, burning, erythema Peeling |
| Hexsel D et al., [23] | 50 Brazilian patients, Solar lentigines- PIH | Triple combination [Fluocinolone acetonide 0.01%, hydroquinone (HQ) 4%, tretinoin 0.05%], suncreens, cryotherapy | Erythema, skin blisters |
| Chandra M et al., [24] | 13 years and above, PIH | 4% HQ, Triple combination (hydroquinone 4%, tretinoin 0.05%, and fluocinolone acetonide 0.01%), Sunscreens | Skin irritation, erythema |
| Monteiro et al., [25] | 60 Patients Indian study, Melasma | 4% Hydroquinone, 0.75% Kojic Acid cream | Skin irritation, erythema |
| Rossi AB et al., [27] | Community based trial, 544 acne patients | Tretinoin 0.04%, Sunscreens | Dermatitis |
| Sarkar R et al., [4] | 40 patients, Indian study, Melasma | 20% Azelaic acid, 2% Kojic acid, 2% Ascorbic acid | Hypo/hyperpigmentation, scarring, keloid formation, secondary infection, allergic reaction, acneiform eruption, persistent erythema, milia and improper healing. |
| Davis EC and Callender VD [30] | 74 patients from darker racial ethnic group with acne- PIH | Tazarotene 0.1% | Erythema, skin peeling, burning, and/or stinging sensation |
| Konda S et al., [9] | 30 South-east Asian women, solar lentigines | Soy extracts | Skin irritation |
In this study, systemic GCs were less often prescribed than topical GCs due to their side effects and calcineurin inhibitors were used as steroid sparing agents, so also in other studies [31-34]. The role of keratolytics in reducing the epidermal melanin content is underpinned by other studies [8-11]. Oral isotretinoin was used in three participants with post acne pigmentation to increase epidermal cell turn over and to facilitate healing; this finding is endorsed by other studies [11,12].
The emerging role of polysiloxanes in PIH (by increasing hydration of stratum corneum and thereby regulates fibroblast production, reduction of collagen synthesis) is also evidenced from a study done by Puri N and Talwar A [35]. The fact that primary disease needs to be treated, before treating residual pigmented areas by administration of oral vitamins and (HCQ) in vitamin B12 deficiency causing hyperpigmentation and PIH (systemic sclerosis) respectively, was vindicated by an Indian and American study [18,36]. In (68.6%) participants, other drugs like emollients, skin protectives and absorbants, oral antioxidants and antihistamines were commonly prescribed as corrigents to provide symptomatic relief comparable to other studies [4,10,33].
In this study, topical agents were more preferred either as topical therapy alone which includes (sunscreens, demelanising agents, GCs, calcineurin inhibitors, keratolytics, polysiloxanes, emollients) or combined therapy (topical+systemic) which includes (systemic GCs, antifibrinolytics, antihistamines, vitamins, antimalarial, antioxidants, retinoids) or FDCs (demelanising agent-HQ 2%+Retinoid-tretinoin 0.025%+steroid-mometasone 0.1%). In participants with melasma, PIH, photomelanosis topical agents were more commonly prescribed either as combined therapy (4% HQ/triple formula/suncreens along with oral tranexamic acid) or FDCs (HQ 2%+mometasone 0.1%+tretinoin 0.025%), and in participants with vitamin B12 deficiency, topical 4% HQ/ Triple formula along with oral vitamins (mecobalamin+pyridoxine+folic acid) were prescribed, whereas for other hyperpigmentary disorders the therapeutic approach was individualised similar to other studies [4,8,10,11,18,37]. According to Chandra M et al., four different agents (HQ, sunscreens, retinoids, steroids) are needed for the optimal treatment of PIH, hence combination creams become an important adjunct to therapy [24]. It improves compliance and reduces adverse effects (irritation due to both HQ and retinoids will be reduced by topical steroids and retinoids reduce steroid-induced atrophy) nevertheless HQ based steroid creams are not be used for prolonged duration [8,24]. As per studies, currently there are no guidelines for the management of other hyperpigmented disorders except melasma, where the first choice is fixed triple combinations, and alternative in case of sensitivity to the ingredients or unavailability of triple combination therapy include dual ingredients (HQ plus GA) or single agents (4% HQ, 0.1% RA, or 20% azelaic acid). Second line therapy include peels either alone or in combination with topical therapy and rarely laser. Combination of topical therapies is more often required to maintain remission status. Due to the diversity of the disorders and the variations of assessing treatment modalities, it is difficult to assess the outcomes [8]. Newer agents like topical methimazole, a potent peroxidase inhibitor is been investigated in melasma as per few studies [12].
In this study, most of the AEs were mild to moderate, self-limiting occasionally requiring symptomatic relief or change in line of therapy. Causality assessment was not performed to any of the AEs. So, the line of therapy was appropriate with least AEs and maximal therapeutic satisfaction/benefit). Similar self-limiting adverse effects like erythema and irritation due to 4% HQ, 0.1% retinoic acid, (0.1% mometasone+2% HQ+0.025% tretinoin), (4% HQ+10% buffered GA+vitamins C and E+sunscreen) and isotretinoin induced skin, lip dryness were reported in other studies and moisturisers were used for symptomatic management in such cases [8,10,11,21,23,24]. Mild erythema, pruritis, burning and contact dermatitis due to 2% kojic acid and 20% azelaic acid was evident in another study, but they resolved on its own in spite of treatment [4,8,11,25,26]. As per studies extended use of topical steroids especially mometasone, a mid- potent steroid beyond 4-8 weeks of therapy has induced acneiform eruption, rosacea and if in case it has been already used once and the patient presents with a relapse, it should be withheld and safer modes of treatment should be employed and monotherapy is to best avoided [8,11,24,38]. Other studies also shows ACD, PIH, and transient hypochromia with HQ therapy and it resolved after discontinuation of HQ.
In this study, there was a significant improvement in QOL probably because of better patient compliance with good understanding of the disease process and the modality of treatment approach. As per the summary of studies pertaining to QOL [Table/Fig-7] triple combination, HQ has improved QOL in patients with melasma, periorbital hyperpigmentation respectively [16,39-43]. Few others state that there was a significant impairment in all domains of QOL before treatment [34,42,43]. There was no loss to follow-up in the present study.
Summary of studies on qualify of life related to hyperpigmentary disorders of skin [16,39-43].
| Authors | Demography | Drugs | Quality Of Life (QOL) |
|---|
| Balkrishnan R et al., [39] | 1290 participants- broad range of races/ethnicities with Melasma | Triple combination (fluocinolone acetonide 0.01%, hydroquinone 4.0%, and tretinoin 0.05%) | Improvement in various aspects of QOL (social life, recreation/leisure and emotional well-being) using Melasma QOL questionnaire (MelasQoL) after 8 weeks of treatment |
| Cestari TF et al., [40] | 300 Brazilian patients, Moderate to severe melasma | Triple combination (fluocinolone acetonide 0.01%, hydroquinone 4.0%, and tretinoin 0.05%) | Significant reduction on MelasQoL-scores (p<0.001) after treatment. Skin appearance botheration, frustration, embarrassment, influence of the disease on interpersonal relationships improved after 8 weeks. |
| Ranjan R et al., [41] | 50 Indian patients, with periorbital hyperpigmentation | 4% hydroquinone, 30% salicylic acid | Pre and post-treatment DLQI was found to be significant (p<0.05) after 12 weeks, medical therapies cause mild to moderate improvement, possess significant impact on QOL. |
| Yadav A and Garg T [42] | 52 Indian Females with acquired hyperpigmentation (PIH, macular amyloidosis, pigmented contact dermatitis, lichen planus pigmentosus) | Impact of disease on QOL, No details on treatment/pre/post-treatment DLQI | DLQI scores (0 to 25)- Moderate effect of QOL-PIH (50%), pigmented contact dermatitis (42.9%) of participants. Very large effect-Macular amyloidosis (40%) and Lichen planus pigmentosus (40%). |
| Arora P et al., [43] | 156 Indian patients, melasma | Impact of disease on QOL. No details on treatment. | High MelasQoL score - 37.5, significant negative impact on QOL (psychosocial and emotional stress) and treatment plan based on it |
| Jiang J et al., [16] | 6 Females - Moderate to severe melasma, Previous history of treatment with 4% HQ cream, tretinoin cream, intense pulsed light (IPL), or a combination of these for at least 3 months prior to their first visit without improvement. | Oral tranexamic acid 325 mg twice daily, triple combination cream (6% hydroquinone, 0.0125% tretinoin, 0.1% dexamethasone once daily), sunscreen lotion. | Self-esteem/self-consciousness, freedom, frustration with costly and ineffective treatments, and improvement in self-esteem, QOL after treatment- (tool- semi-structured interviews). |
Limitation(s)
The available therapeutic approaches were not compared for efficacy, tolerability and safety. The analysis of the cost effectiveness was not performed.
Conclusion(s)
Topical therapy was effective in most of the participants in the study, systemic agents were used as an adjuvant for better therapeutic response, symptomatic relief and to treat underlying infections. The most commonly prescribed medication includes HQ, tranexamic acid and triple formula. The AEs observed were well tolerated and self-limiting with a few exceptions like acneiform eruption, rosacea with topical GCs. The QOL significantly improved in terms of participant acceptability and compliance to the available therapeutic approach. A number of comparative studies are deemed necessary to establish, standard guidelines in the management of hyperpigmentary disorders using various therapeutic approaches.
*On Multidrug therapy for Multibacillary type of leprosy (MDT-MB)
*n=Total number of subjects receiving the particular class of drug;
N=Number of subjects receiving the individual drug. One or more drugs of same class or same drugs of different formulation were used in same/one subject;
†Concentration is expressed as percentage, based on w/w (weight/weight)
*One or more side-effects were seen in same subject