Pregnancy is a physiological state associated with various alterations in metabolic, biochemical, physiological, haematological and immunological processes. If such changes are exaggerated, they can lead to serious complications during pregnancy [1]. Pre-eclampsia is one such common and serious complication of pregnancy and is associated with increased maternal and perinatal morbidity and mortality [2]. Pre-eclampsia is characterised by high maternal systolic Blood Pressure (BP) ≥140 mmHg and/or diastolic BP ≥90 mmHg measured with associated proteinuria (protein excretion of ≥300 mg in 24 hours urine) that develops after 20 weeks of gestation in previously normotensive women [3]. Pre-eclampsia if untreated may succeed in eclampsia, oliguria, visual disturbances, HELLP syndrome (Haemolytic anaemia, Elevated Liver enzymes and Low Platelet count), pulmonary oedema and foetal growth restriction [4]. Despite its prevalence, severity and considerable research, the pathophysiology of pre-eclampsia is not fully understood and the exact cause is still unknown.
Alteration in various haematological and biochemical parameters is observed in patients with pre-eclampsia and they are often considered as predictors for development of the disease. Among the biochemical parameters serum calcium, magnesium, vitamin D, thyroid hormone levels, serum copper and serum iron have been found to be associated with the occurrence of pre-eclampsia [3,5,6].
Increased oxidative stress contributes substantially in the pathogenesis of pre-eclampsia [5,7]. The presence of transition metal ions, particularly iron can play a crucial catalysing role in the production of Reactive Oxygen Species (ROS) via formation of hydroxide (OH-) and the very reactive hydroxyl radicals (HO) by the Haber-Weiss and Fenton reactions, and can initiate the process of lipid peroxidation which eventually may result in endothelial cell damage [8,9]. Literature suggests that an increased iron and ferritin concentration is associated with a higher risk of pre-eclampsia [9]. Some reports even suggest that prophylactic iron supplementation can be harmful to pregnant women who are otherwise not iron deficient [10]. Excess of iron in context of availability or storage can therefore promote or enhance oxidative stress. That is why, perhaps, experts worldwide diverge on whether iron supplementation should be a routine during pregnancy [11-13].
In developing countries including India, iron deficiency is presumed without serum ferritin estimation and iron supplementation is regularly prescribed to pregnant women. Although there are many studies conducted in the past in India and abroad [14-16] except few, most of these studies do not reveal a clear picture of relationship among iron and associated parameters in pre-eclampsia. These studies have shown elevated ferritin levels in pre-eclamptic subjects but failed to conclude whether levels among pre-eclamptic subjects was due to iron toxicity or as a marker of acute phase response [5,17]. Also, very few studies have been published on iron supplemented population or have clearly defined whether the population was supplemented with iron doses or not [18-20]. Moreover, disparity in socio-economic, demographic and lifestyle characteristics among populations of different regions impresses upon the need of carrying similar studies to draw valuable inferences [21].
Considering aforesaid facts and to better correlate iron related parameters with pre-eclamptic characteristics, the present study was designed to: a) Analyse the iron related parameters among iron supplemented subjects; b) Compare the levels in cases with controls to establish the differences; c) Calculating the associated odds and correlation of the parameters with pre-eclamptic occurrence; and d) Analyse the results in context of previous observations in similar studies.
Materials and Methods
This prospective, observational, case-control study was conducted in the Department of Biochemistry and Department of Obstetrics and Gynaecology, Jaipur National University Institute of Medical Sciences and Research Centre (JNUIMSRC), Jaipur, Rajasthan, India, for a period of one year (February 2019-January 2020). The study was approved by Institutional Ethics Committee (IEC) vide reference no. (JNUIMSRC/IEC/2019/74) dated 21-1-2019. The ethical approval was in accordance with Helsinki Guidelines.
Sample size: A minimum sample size of 100 patients in each group was calculated using effect size of 0.4 and with user constant type I error α=0.05 and type II error β=0.2 by using G power software (3.1.9.7).
The study protocol and the objectives of the study were explained to the enrolled subjects and their written informed consent was obtained from all the participants. After a thorough clinical examination, a total of 200 pregnant females were selected: 100 in pre-eclampsia group (case group); 100 in normotensive group (control group), in the third trimester and between 18-45 years, visiting Gynaecology Out Patient Department (OPD) or admitted in JNUIMSRC were enrolled in the study. Equal number of primigravid and multigravid women (age and gestational age matched) were included in both case and control groups. The demographic details, obstetric history and relevant medical history of all the study subjects were recorded in a predesigned performa. Investigations were conducted in third trimester of pregnancy.
All the enrolled study participants received oral elemental iron (60 mg/day) from the beginning of the second trimester and supplement continued throughout the gestation as a measure for preventing iron deficiency anaemia.
Inclusion criteria:
Case group (Pre-eclamptic group): The case group included pregnant women having BP ≥140/90 mmHg measured on two occasions with an interval six hours in between, having proteinuria ≥1+ with dipstick (≥which corresponds to 300 mg/24 hours urine). Proteinuria was measured by taking two random midstream urine samples for dipstick after a gap of four hours.
Control group (Normotensive group): The control group included normotensive pregnant women (BP ≤140/90 mmHg) and without any evidence of proteinuria.
Exclusion criteria: Pregnant women, transferred with pack red blood cells in last 30 days, previously diagnosed with haemochromatosis, and/or complications like diabetes mellitus, chronic hypertension, chronic renal disease, chronic liver disease, cardiovascular disease, endocrinal disorder, autoimmune disease, hyperuricaemia, genital malignancies, blood discrasias, acute infections, and those on anticonvulsant drugs were excluded from the study. Among the multigravida any history of hypertension, trophoblastic disease, placental abruption, malformed foetus, history of pre-eclampsia, metabolic disorders or convulsions were ruled out. Also, child-bearing women with history of substance abuse or smoking were excluded.
Study Procedure
Procedures: The digital sphygmomanometer was used for measuring BP from the left arm in sitting position after five minutes rest. The systolic and diastolic readings were recorded by calculating an average of three readings taken after a gap of five minutes.
Sample collection: Blood samples were collected from the subjects and were coded to avoid possible bias. A total of five mL venous blood was collected from each subject under aseptic conditions in a sterile syringe. Thereafter, blood sample was transferred into clean and dry vial without anticoagulant, was allowed to clot spontaneously and then centrifuged at 3000 revolution per minutes (rpm) for 15 minutes to extract serum. The extracted serum was collected in microcentrifuge tubes and was used for estimation of following biochemical parameters: serum iron, serum ferritin, sTFR and TIBC.
Freshly voided, random, midstream urine sample (15-20 mL) was collected on two different occasion’s at least (four hours apart) in clean, wide mouthed, leak proof containers for urinalysis.
Biochemical investigations: Among the biochemical parameters; serum iron, TIBC and sTFR were analysed using a fully automated analyser by RANDOX, Crumlin, UK, and serum ferritin was analysed using a fully automatic analyser by Johnson and Johnson, VITROS®ECiQ, Immunodiagnostic system, Ortho Clinical Diagnostics, New Jersey, USA.
Proteinuria was measured by Combur10 test® M strips by Roche and analysed by using semi-automatic Cobas u 411 urine analyser by Roche diagnostics, Germany, and the samples were graded as 1+, 2+ or 3+.
Statistical Analysis
The statistics analysis was conducted using Statistical Package for the Social Sciences (SPSS) 26.0. Kolmogorov-Smirnov test (N=200) was used to assess normality of parameters concerned. None of the parameters followed normal distribution and thus were expressed as Median IQR and compared by using Mann-Whitney U test. The correlation was assessed by Spearman’s Rank correlation and categorical variables were assessed by Chi-square tests. The p-value <0.05 was considered statistically significant.
Results
Demographic details and the general characteristics of case and control groups are compared in [Table/Fig-1]. Both the groups; case group as well as control group, were comparable regarding age, gestational age and parity. Median (IQR) of systolic and diastolic BP of the cases were found to be significantly higher than controls (p<0.0001, respectively). [Table/Fig-1] also depicts socio-economic, demographic as well as dietary habits of pre-eclamptic cases and normotensive controls. There was no significant difference with the cases and controls with respect to the aforesaid parameters.
Demographic details and the general characteristics of pre-eclamptic cases (case group) and normotensive controls (control group).
| Variables | Cases (n=100) Median (IQR) | Controls (n=100) Median (IQR) | p-value |
|---|
| Age (years) | 23 (22-26) | 24 (22-26) | 0.64 |
| Gestational age (weeks) | 37 (36-39) | 38 (37-39) | 0.122 |
| BMI (kg/m2) | 24 (18-28) | 23 (21-25) | <0.0001* |
| Systolic BP (mmHg) | 150 (145-162) | 120 (110-126) | <0.0001* |
| Diastolic BP (mmHg) | 101 (97-110) | 80 (70-81) | <0.0001* |
| Primigravida n (%)Multigravida n (%) | 61 (61%)39 (39%) | 65 (65%)35 (35%) | 0.90 |
| Rural n (%)Urban n (%) | 61 (61%)39 (39%) | 60 (60%)40 (40%) | 0.30 |
| Low income n (%)Middle income n (%) | 59 (59%)41 (41%) | 41 (41%)56 (56%) | 0.30 |
| Vegetarian n (%)Non vegetarian n (%) | 52 (52%)48 (48%) | 44 (44%)56 (58%) | 0.60 |
| Iron supplementation | 60 mg/day | 60 mg/day | NA |
BMI: Body mass index; BP: Blood pressure; *Significant Mann-Whitney U test for age, gestational age, BMI, systolic BP and diastolic BP. Chi-square test for primigravida and multigravida, rural and urban, low and middle income, vegetarian and non vegetarian sub-groups
[Table/Fig-2] depicts the median (IQR) values of serum iron, TIBC, serum ferritin and sTFR among both the study group. The difference of median (IQR) of serum iron levels and TIBC between cases and controls were statistically insignificant. Median IQR levels of sTFR (mg/dL) in cases were significantly low in comparison to controls (8.5 (7-9) vs 8.9 (8-10, p=0.023). Also, median IQR of serum ferritin levels (ng/mL) of the cases were found to be significantly higher than the controls (41(30-70) vs. 24 (17-44), p<0.001). Moreover, participants with serum ferritin levels >40 (ng/mL) were associated with three times increased risk of PE (Odds ratio: 3, p=0.004, 95% Cl=1.571-5.157) [Table/Fig-3].
Comparison of serum iron, TIBC, serum ferritin and sTFR among pre-eclamptic cases (case group) and normotensive controls (control group). p-values calculated by Man-Whitney U test.
| Variables | Cases (n=100) Median (IQR) | Controls (n=100) Median (IQR) | p-value |
|---|
| Iron (μg/dL) | 76 (47-117) | 79 (58-95.5) | 0.95 |
| TIBC (μg/dL) | 485 (404-523) | 493 (448-544) | 0.062 |
| Ferritin (ng/mL) | 41 (30-70) | 24 (17-44) | <0.001* |
| sTFR (mg/dL) | 8.5 (7-9) | 8.9 (8-10) | 0.023* |
sTFR: Serum transferrin receptor; TIBC: Total iron binding capacity; *Significant
Distribution of cases and controls according to serum ferritin levels (below and above 40 ng/mL concentration) and the associated odds ratio (Cases: n=100, Controls: n=100).
| Variables | Cases, n (%) | Controls, n (%) | Odds ratio |
|---|
| Ferritin >40 (ng/mL) | 50 (50) | 26 (26) | 3 |
| Ferritin <40 (ng/mL) | 50 (50) | 74 (74) |
For comparative analysis of Ferritin: χ2=12.2, df=1, p=0.004. 95% Cl=1.571-5.157, Cl: Confidence interval
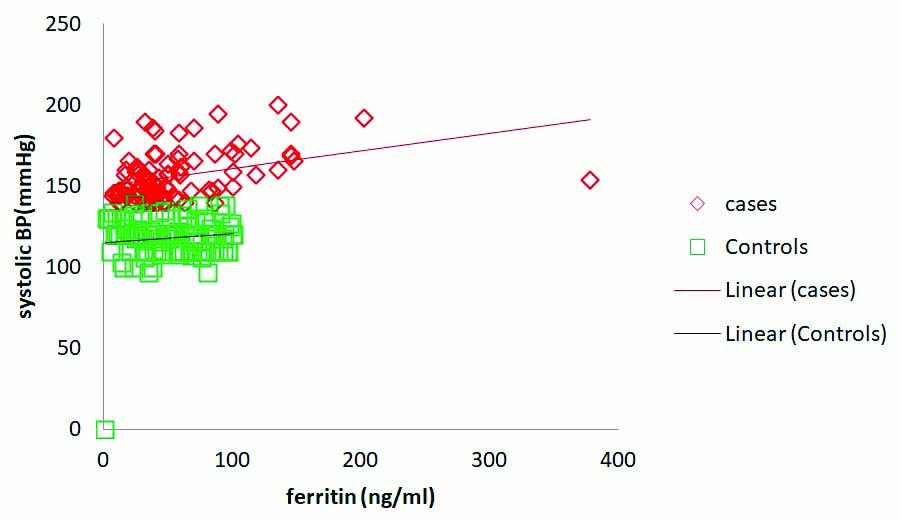
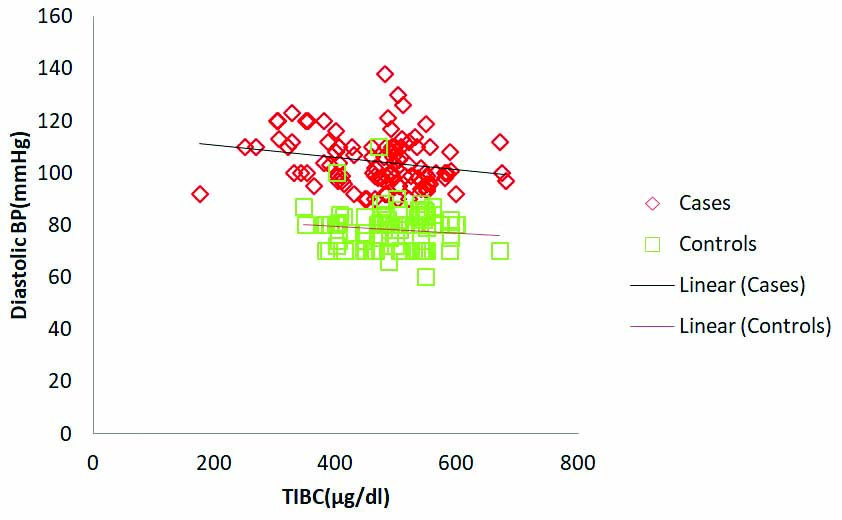
Serum ferritin levels in pre-eclamptic cases significantly correlated positively with systolic BP (r=0.37, p=0.001). However ferritin levels in controls did not show significant correlation with systolic BP (r=0.043, p=0.67) [Table/Fig-4,5 and 6]. TIBC in case group showed significant negative correlation with diastolic BP (r=-0.238, p<0.05) and insignificant correlation was observed in controls (r=-0.057, p=0.57) [Table/Fig-7]. The sTFR in pre-eclamptic cases and controls showed insignificant negative correlation with systolic and diastolic BP [Table/Fig-4]. In controls, ferritin, TIBC and sTFR did not show significant correlation with either systolic/diastolic BP [Table/Fig-5] whereas in cases ferritin failed to show any significant correlation with diastolic BP and TIBC with systolic BP [Table/Fig-4].
Correlation coefficients of variables with systolic and diastolic Blood Pressure (BP) in pre-eclamptic cases. p-values calculated by Spearman rank correlation test. *Significant
| Variables | Systolic blood pressure | Diastolic blood pressure |
|---|
| Correlation coefficient | p-value | Correlation coefficient | p-value |
|---|
| Ferritin | 0.37 | 0.001* | 0.16 | 0.113 |
| sTFR | - 0.08 | 0.123 | - 0.14 | 0.182 |
| TIBC | - 0.132 | 0.19 | - 0.238 | 0.017* |
Correlation of variables with systolic and diastolic Blood Pressure (BP) in controls. p-values calculated by Spearman’s rank correlation test.
| Variables | Systolic blood pressure | Diastolic blood pressure |
|---|
| Correlation coefficient | p-value | Correlation coefficient | p-value |
|---|
| Ferritin | 0.043 | 0.672 | 0.092 | 0.36 |
| sTFR | - 0.091 | 0.36 | 0.129 | 0.20 |
| TIBC | - 0.006 | 0.95 | -0.057 | 0.57 |
Serum ferritin levels were observed to correlate positively with systolic Blood Pressure (BP). Observed correlation was significant in pre-eclamptic cases (r=0.37, p=0.001). No significant correlation was observed between serum ferritin and systolic BP in control subjects (r=0.043, p=0.67).
TIBC levels were observed to correlate negatively with diastolic Blood Pressure (BP). Observed correlation was significant in pre-eclamptic cases (r=-0.23, p=0.017). No significant correlation was found between TIBC and diastolic BP in control subjects (r=-0.057, p=0.57).
Discussion
Iron deficiency is one among the most common causes of anaemia in pregnancy and hence in many countries, particularly in developing nations, routine iron supplementation to pregnant women has been a public health intervention for a long time [22]. There are many studies supporting the benefit of this practice, however, some reports suggest that prophylactic iron supplementation may be harmful to pregnant women who are not iron deficient [10]. Thus, iron intake, transport, distribution and storage needs to be organised accurately to balance these double characteristics of essentiality and toxicity, which is critical for normal body homeostasis [10,23]. Also, the central organ regulating pregnancy, the placenta, acts as one of the chief sources of iron to the foetus. Due to its potential redox activity, iron can be toxic as it catalyses the formation of ROS, which eventually can result in a state of oxidative stress, mainly in the second trimester of pregnancy. Excess iron supplementation can result in saturation of proteins to which it binds (ferritin and transferrin), leaving iron in its free form in the bloodstream which can lead to oxidative stress with potentially damaging effects in mother and the newborn.
In the present study, serum iron levels were observed to be similar in cases and controls. Similar findings were reported by Gutierrez-Aguirre CH et al., [5]. However, contradictory observations have been reported by Shaji Geetha N et al., and Jana SM et al., where serum iron levels were observed at increased levels in cases [18,20]. These differences could indicate compromised iron intake in the population under present study.
Present study results regarding TIBC levels in pre-eclamptic cases were similar to controls. Similar findings were observed in the study conducted by Jana SM et al., [20]. However, the reliability of TIBC as an indicator of iron availability to the tissues is questionable since its levels show fluctuations in physiological conditions like diurinal rythm [19].
In the present study, serum ferritin levels were higher in pre-eclamptic cases. Similar finding were also observed in the studies of Shaji Geetha N et al., Elshahat AM et al., and Mandal D et al., [18,19,24]. The serum ferritin levels <15 ng/mL serve as an indicator of depleted iron stores [25]. However, in the present population, serum ferritin levels were comparatively high thus ruling out the possibility of iron deficiency anaemia.
Increased serum ferritin can also be due to acute phase response, a phenomenon observed in pre-eclamptic cases. However, past studies have revealed that acute phase response is not the sole cause for increased ferritin levels in pre-eclampsia [9]. This complication lead us to sTFR that serves as a more reliable indicator in determining iron status as its levels are not altered because of acute phase response [26]. Present study results show lower sTFR levels in cases. This further indicates that iron supplementation could be a possible cause of elevated serum ferritin levels in pre-eclampsia.
Previous studies have shown increased risk of complications like preterm birth among pre-eclamptic women having serum ferritin levels >40 ng/mL [5]. In the view of above facts, in present study subgroup analysis considering the participants having serum ferritin >40 ng/mL. Present study results revealed greater number of participants in case group having serum ferritin levels >40 ng/mL (50% vs 26%). Moreover, the study conducted by Elshahat AM et al., evaluated a cut-off value of serum ferritin >43 ng/mL as a criteria for prediction of pre-eclampsia [19]. In this context, present study revealed that participants having serum ferritin levels >40 ng/mL had about three times increased risk of pre-eclamptic occurrence.
A positive correlation of serum ferritin with BP and a negative correlation of TIBC with BP further strengthen the association between iron status and the contributions it can make in aetiopathogenesis of pre-eclampsia. Similar findings were also observed in a study conducted by Biswas S et al., [27]. Contrary to these findings Kanagal DV et al., did not find any significant correlation of the above paramaters with systolic or diastolic BP among pre-eclamptic cases [17]. A small sample size of their study could have been the possible cause attributed to this non correlation.
Limitation(s)
This study, with larger sample size, can be extended in the form of multicentric study, in order to include other populations with demographic variations. Also, similar studies could be performed on non anaemic population, population with predominant iron deficiency anaemia and that with iron mediated toxicity. These case-control studies are also needed to be supplemented with more cohort studies in order to establish the iron contribution in pre-eclamptic aetiopathogensis.
Conclusion(s)
Higher serum ferritin levels and lower sTFR values were observed in cases. A correlation of BP with ferritin and that with TIBC is also established. Present study results show lower sTFR levels in cases. These observations indicate that iron supplementation could lead to enhanced iron status in pre-eclamptic cases compared to controls. Although, none of the iron related parameters in this study could be related to iron overload, yet an unchecked and unmonitored iron supplementation could further increase a possibility of iron mediated added complications in pre-eclampsia. Present study results, in combination with previous studies from others, hereby suggest that cautionary tests for estimation of serum ferritin and sTFR levels should be conducted to assess iron status in pre-eclampsia before prescribing or continuing with iron supplementation. This would help to prevent any possibility of iron overload in pre-eclamptic patients. The assessment would further help clinicians to restrict/lower the prescribed dose of iron supplements. Authors however, also believe that in situations where screening for serum ferritin and sTFR levels is not possible, iron supplementation during pregnancy should be kept continued, especially in population with predominant iron deficiency.
BMI: Body mass index; BP: Blood pressure; *Significant Mann-Whitney U test for age, gestational age, BMI, systolic BP and diastolic BP. Chi-square test for primigravida and multigravida, rural and urban, low and middle income, vegetarian and non vegetarian sub-groups
sTFR: Serum transferrin receptor; TIBC: Total iron binding capacity; *Significant
For comparative analysis of Ferritin: χ2=12.2, df=1, p=0.004. 95% Cl=1.571-5.157, Cl: Confidence interval