Chronic viral hepatitis is a disease that endlessly triggers inflammation and necrosis in the liver parenchyma. If the inflammation is allowed to continue, hepatocellular necrosis results in extensive fibrosis that finally leads to liver cirrhosis. Among the various aetiologies, hepatitis B and C commonly results in a chronic disease with liver fibrosis representing the organ’s final response to injury. The prevalence rate of hepatitis B and C infection in the Indian population has been estimated to be 3.7% and 1%, respectively [1,2]. Of these patients, 5-10% of Hepatitis B (HBV) infection and 75-85% of Hepatitis C Virus (HCV) infection tend to become chronic [3,4].
Liver fibrosis is a progressive disorder which if diagnosed early and staged precisely, allows early clinical intervention to slow down the progression to end-stage cirrhosis. Although end-stage fibrosis is conventionally considered irreversible, newer studies suggests that recovery from cirrhosis may be possible. It has been reported that the combination of pegylated polyethylene glycol (PEG)-interferon and ribavirin significantly reduces the rate of progression of fibrosis in early stages in patients with Hepatitis C [5-7]. Many therapeutic agents targeting the fibrogenesis pathways have been studied, particularly the Transforming Growth Factor-β (TGF-β1) and hepatic stellate cells (the main collagen producing cells in the liver) [8]. Correct assessment of the severity of fibrosis is thus a pre-requisite to correctly stage and prognosticate the Chronic Liver Diseases (CLD) and plan appropriate therapy.
Biopsy remains the reference standard for the estimation of liver fibrosis. However, it is invasive and evaluates only a minuscule part of the liver parenchyma [9]. Conventional imaging techniques can identify the presence of hepatic fibrosis but they cannot assess the degree of fibrosis. This has spurred extensive interest in the development of non invasive serological tests like Aspartate-Aminotransferase Platelet Ratio Index (APRI) and advanced imaging modalities like Shear Wave Elastography (SWE). SWE can assess the degree of stiffness (both semi quantitatively and quantitatively) that parallels liver fibrosis in patients with chronic viral hepatitis [10-15]. Aspartate-APRI is an indirect biochemical marker presently used for the estimation of liver fibrosis in chronic viral hepatitis. Its value tends to increase with the severity of liver fibrosis [16].
There is a scarcity of literature defining the role of SWE in the assessment and grading of liver fibrosis in chronic viral hepatitis amongst the Indian population. Furthermore, little data exists which explores the relationship between SWE and APRI in this clinical setting. This study endeavoured to determine, if non invasive techniques like SWE and APRI are reliable diagnostic tools for detecting and grading hepatic fibrosis and if they can replace liver biopsy for the purpose of clinical follow-up.
Materials and Methods
This case control study was approved by the Institute Ethics Committee (S.No. IEC/VMMC/SJH/Thesis/October/2017-202) and was conducted over a period of 18 months, from October 2017 to March 2019, at Vardhaman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. All patients gave informed consent to participate in the study.
Sample size calculation: Sample size was calculated using following formula [17]:
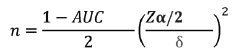
Where, Zα is value of Z at two-sided alpha error of 5% and δ is 0.085
On the basis of a previous study by Sande JA et al., authors have taken the AUROC (Area Under the Receiver Operating Characteristics) using SWE alone as 0.91 compared to 0.78 for using the APRI score alone [18]. In the referenced study, SWE and APRI score differentiated F0-1 from F2-4 with an AUROC of 0.920. Taking these values as reference with δ of 0.085, and 5% level of significance, we could calculate a sample size of 59 patients. Hence, the sample size of 60 was considered.
Inclusion criteria: Patients with chronic hepatitis C, characterised by persistence of either HCV antibodies or Hepatitis C Virus-Ribonucleic Acid (HCV-RNA) in serum for more than six months after initial diagnosis and Chronic hepatitis B, characterised by the persistence of Hepatitis B surface antigen and/or HBV-DNA in serum for more than six months after initial diagnosis were included in study [3,4].
Exclusion criteria: Patients with alcoholic liver disease (those with significant alcohol intake, i.e., 40-80 gm/day in males and more than 20 gm/day in females for at least 10 years), those with hepatocellular carcinoma, pregnant and breast-feeding patients were excluded from the study.
Out of 120 subjects enrolled in the study, biopsy could not be performed in 10 cases so these cases and their matched controls were excluded from the statistical analysis. Amongst the 100 subjects who were analysed, 50 cases of chronic viral hepatitis and 50 case-matched healthy volunteers as controls were taken.
Procedure
Shear Wave Elastography (SWE): SWE was performed using a 1-5 MHz curvilinear transducer (Philips iU22 xMATRIX Ultrasound Machine, Philips Healthcare, Andover, MA). Ultrasound scanning of the right lobe of the liver was performed, to select the location where the liver thickness becomes at least 60 mm [19]. After appropriate breath-hold, examination was done through the right inter-costal spaces and measurements were taken by placing rectangular Regions of Interest (ROI) 1-2 cm under the liver capsule, avoiding any major vascular structure, biliary radicals, and rib shadowing [Table/Fig-1]. Six measurements were taken for each patient and their mean value was expressed in kilopascals (kPa). Ultrasound elastography measurements taken in chronic hepatitis patients are shown in the [Table/Fig-2].
Image shows liver stiffness measured with 2D Shear wave elastography of a 38-year-old healthy female volunteer, through right inter-costal view after appropriate breath hold. Rectangular Regions of Interest (ROI) box was placed 1-2 cm under liver capsule, avoiding any major vascular structure, biliary radicals, and rib shadowing.
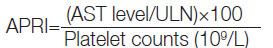
a) Grey scale ultrasound SWE image shows liver stiffness of chronic viral hepatitis patients with corresponding histopathological grades on biopsy; SWE=7.27±1.83 kPa, mean stiffness 7.4 kPa (METAVIR grade=F1); b) Grey scale ultrasound SWE image shows liver stiffness of chronic viral hepatitis patients with METAVIR F2 grade fibrosis on biopsy; SWE=7.78±1.13 kPa, mean stiffness 8 kPa (F2); c) Grey scale ultrasound SWE image shows liver stiffness of chronic viral hepatitis patients with METAVIR F3 grade fibrosis on biopsy; SWE=8.54±3.85 kPa, mean stiffness 8.4 kPa (F3); d) Grey scale ultrasound SWE image shows liver stiffness of chronic viral hepatitis patients with METAVIR F4 grade fibrosis on biopsy; 19.36±6.31 kPa, mean stiffness 17 kPa (F4).
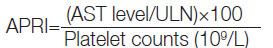
Parameter: Age, sex, weight, height, and body mass index were recorded for each subject at the time of the study along with a brief medical history and physical examination findings. Percutaneous ultrasound-guided liver biopsy was performed in all patients of chronic viral hepatitis after obtaining a written consent and excluding those with ascites. Serum Aspartate Aminotransferase (AST), serum Alanine Aminotransferase (ALT), serum ALT, platelet count, Prothrombin Time and International Normalised Ratio (PT/INR) were done for all patients before proceeding for a liver biopsy. Reference values used for APRI and SWE for estimating the degree of liver fibrosis is shown in [Table/Fig-3] [20,21]. APRI was calculated using the following formula [16]:
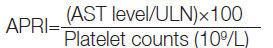
Reference values used for APRI [19] and SWE [15] for estimating the degree of liver fibrosis.
| METAVIR score [21] (Liver biopsy fibrosis grades) | F0 | F1 | F2 | F3 | F4 |
|---|
| SWE (kPa) | <7.1 | <7.8 | <8 | <11.5 | >11.5 |
| APRI | <0.5 | 0.5-1.5 | >1.5 | | |
SWE: Shear wave elastography; APRI: Aminotransferase platelet ratio index; kPa: Kilo-pascal
Biopsies were done within one week following the SWE examination. METAVIR scoring system was used for histopathological grading of liver fibrosis [21,22].
F0=No fibrosis
F1=Portal fibrosis without septa
F2=Portal fibrosis with rare septa
F3=Numerous septa, not cirrhosis
F4=Cirrhosis
Statistical Analysis
Categorical variables were presented in number and percentage and continuous variables were presented as mean±standard deviation and median. The normality of data was tested by the Kolmogorov-Smirnov test. If the normality was rejected then the non-parametric test was used. Quantitative variables were compared using the Independent t-test/Mann-Whitney Test (when the data sets were not normally distributed) between the two groups and one-way ANOVA/Kruskal Wallis test was used for comparison between more than two groups. Qualitative variables were correlated using the Chi-square test/fisher’s-exact test. The Receiver Operating Characteristic (ROC) curve was used to find out the cut-off point of the mean of SWE Values and APRI for predicting F0 vs F1-F4, F0-1 vs F2-4, F0-2 vs F3-F4, F0-F3 vs F4. Univariate and multivariate logistic regression was used to find out significant risk factors of F1-F4, F2-F4, F3-F4, and F4. A p-value of <0.05 was considered statistically significant. The analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
Among the 120 subjects enrolled in the study, 60 were diagnosed cases of chronic viral hepatitis, and 60 were healthy volunteers who were age, sex, and body mass index matched to that of cases and negative for hepatitis B and C serology. The average height, weight and Body Mass Index (BMI) of the participants were 166.9 cm, 63.1 kg and 22.7 kg/m2, respectively. The age distribution of cases and controls (n=220) were analysed. The mean age was found to be 38.3±10.82 years. The youngest subject in the study group was 14-year-old and the oldest was 56-year-old. Among 60 cases of chronic viral hepatitis, the most prevalent viral infection was hepatitis B which was noted in 41 patients (68.3%), followed by hepatitis C in 14 patients (23.3%) and hepatitis B and C co-infection in five patients (8.3%). Chronic viral hepatitis was most prevalent among the housewives 14 patients (23%) followed by health care workers (10 cases 16.6%) and farmers seven patients (11.6%). The most common source of infection was parenteral drug administration in 19 cases (31.6%), direct contact with a person with hepatitis in 13 cases (21.6%), source of infection was unknown in 12 cases (20%), history of blood transfusion in 11 cases (18.3%), organ transplant in three cases (5%), and haemodialysis in two cases (3.3%).
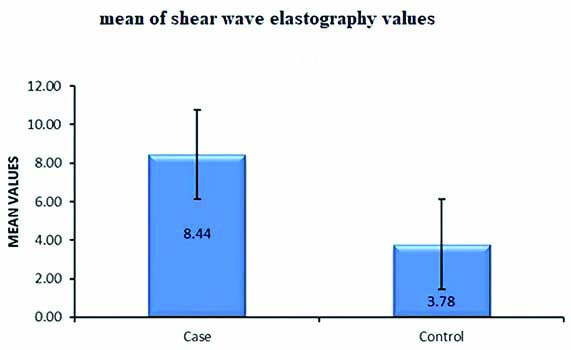
The mean age among normal subjects (n=60) was 38.8±10.6 years (mean±2SD). Among the 60 controls, 35 were male and 25 were female. The mean liver stiffness (shear modulus) for normal subjects was found to be 3.78±0.76 kPa [Table/Fig-4]. The mean liver stiffness for males was found to be 3.75 kPa while that for females was 3.88 kPa. There was no significant statistical difference between liver stiffness values neither between males and females (p-value=0.552, α=0.05) nor between different age groups (p-value=0.429, α=0.05) among normal subjects [Table/Fig-5]. The overall mean SWE value for the cases (n=60) was 8.44±4.01 kPa. Among cases (n=60) the mean liver stiffness value in males and females was 8.32 kPa and 8.63 kPa respectively. The mean SWE and APRI values among cases (n=50) corresponding to the histopathological grading of fibrosis is shown in the [Table/Fig-6].
Mean values of shear wave elastography among cases and healthy volunteers.
Distribution of SWE value among various age groups in study subjects and their mean elastography values.
| Variables | Age (years) | ≤20 | 21-30 | 31-40 | 41-50 | 51-60 | Total | Test value (p-value) |
|---|
| Control | Number of controls | 6 | 10 | 14 | 24 | 6 | 60 | @f 1.34 (0.268) |
| Mean | 4.13 | 4.18 | 3.62 | 3.67 | 3.81 | 3.80 |
| SD | 0.57 | 0.67 | 0.85 | 0.69 | 0.93 | 0.75 |
| Median | 4.32 | 4.44 | 3.53 | 3.43 | 3.95 | 3.58 |
| Min | 3.42 | 2.68 | 2.60 | 2.40 | 2.68 | 2.40 |
| Max | 4.82 | 4.82 | 4.82 | 4.77 | 4.82 | 4.82 |
| Case | Number of cases | 6 | 10 | 14 | 24 | 6 | 60 | $f 1.45 (0.228) |
| Mean | 6.76 | 6.49 | 9.67 | 8.89 | 8.79 | 8.45 |
| SD | 2.01 | 1.95 | 4.09 | 4.42 | 3.19 | 3.80 |
| Median | 7.48 | 7.30 | 7.90 | 7.90 | 8.09 | 7.77 |
| Min | 2.67 | 2.88 | 4.55 | 4.12 | 4.92 | 2.67 |
| Max | 7.92 | 8.48 | 19.25 | 21.93 | 13.93 | 21.93 |
| Comparison between cases and controls | #t 9.27 (<0.001) |
@Comparision of mean values of liver elasticity value among age group of control with one-way ANOVA test; $Comparison of mean values of liver elasticity value among age group of case with one-way ANOVA test; #Comparision of mean values of liver elasticity value among cases and control are calculated with independent t-test.
Liver biopsy grading and corresponding mean cut-off values of SWE and APRI.
| Liver biopsy results (n=50) | F0 (n=9) | F1 (n=12) | F2 (n=9) | F3 (n=11) | F4 (n=9) |
|---|
| Mean APRI | 0.34 | 0.72 | 0.81 | 0.98 | 1.91 |
| Median of SWE values | 4.48 | 7.16 | 8.08 | 8.44 | 16.01 |
| Mean of SWE values | 4.48±1.05 | 7.16±0.75 | 8.08±1.05 | 8.44±0.47 | 16.01±3.78 |
Discussion
Viral hepatitis is a disease that causes inflammation and necrosis in the liver parenchyma ad infinitum. If the hepatocellular necrosis continues, it would cause fibrosis and finally cirrhosis [18,23,24]. Increased incidence of liver fibrosis will continue to burden the health care system as there is a rise in associated morbidity and mortality [2]. Biopsy, an invasive investigation, continues to be the gold standard to assess the degree of liver parenchymal damage and the amount of liver fibrosis [25]. However, liver biopsy in every chronic viral hepatitis patient remains a controversial choice and is impractical to repeat on every follow-up especially due to its invasive, resource-intensive and time consuming nature. Liver biopsy is fraught with the risk of major complications like excessive bleeding, pneumothorax, biliary peritonitis and rarely death [26] . The need of the hour is to devise non invasive techniques to accurately diagnose and grade liver fibrosis and for post-treatment follow-up of these patients. Currently, SWE and APRI are considered front runners to assess the degree of fibrosis in chronic viral hepatitis so that liver biopsy can be reserved for selected cases. SWE is a reliable modality for the quantitative evaluation of liver parenchymal stiffness in vivo. It is non invasive, easily reproducible, and cost-effective compared to liver biopsy [26]. The present study was an endeavour to assess the change in liver parenchymal stiffness quantitatively by SWE, to assess the impaired liver function by APRI, to know their role in the grading of liver fibrosis and also to correlate with histopathological scoring (METAVIR).
In the present study, mean liver stiffness in healthy volunteers was found to be 3.78±0.75 kPa. This result is consistent with normal stiffness values shown in previous studies [27-30]. Amongst normal subjects, mean liver stiffness in males (3.89kPa) was slightly higher as compared to females (3.69kPA) which concurs with the study conducted by Huang Z et al., where normal liver stiffness was greater in males (5.45 kPa) than in females (4.89 kPa) [Table/Fig-7] [29]. No correlation between age and liver stiffness was found which is consistent with previous studies [27-29].
Comparison of mean liver stiffness among healthy volunteers in the present study and in previous studies [27-29].
| Present study | Previous studies |
|---|
| Mean liver stiffness- 3.78±0.76 kPa | Mean liver stiffness- 3.3±2.1 kPa [27] |
| Mean liver stiffness- 2.6-6.2 kPa [28] |
| Mean liver stiffness- 5.10±1.02 kPa [29] |
| Mean liver stiffness in females- 3.88 kPA | Mean liver stiffness in females- 4.89±0.96 kPa [28] |
| Mean liver stiffness in males- 3.75 kPa | Mean liver stiffness in males- 5.45±1.02 kPa [28] |
Out of total 60 cases of chronic hepatitis, 50 underwent ultrasound guided percutaneous liver biopsy out of which nine cases were of grade F0, 12 were of grade F1, nine were of grade F2, 11 were of grade F3 and 9 were of grade F4 based on histopathology scoring (METAVIR). The mean SWE values corresponding to the histopathological grading of fibrosis i.e., F0, F1, F2, F3 and F4 was found to be progressively increasing with the increasing grades of fibrosis and the mean cut-off values were comparable to the cut-off values given by Sporea I et al., and is slightly higher than those found in the studies by Sande JA et al., and Pradeep JA et al [Table/Fig-8] [15,18,31]. This increase in liver stiffness is secondary to the accumulation of extracellular matrix, resistance to the flow of fluids and other changes triggered by parenchymal inflammation [32,33].
Comparison of mean elasticity values for corresponding histological grades of liver fibrosis obtained in the present study with previous studies [15,18,31].
| Present study | Sande JA et al., [18] | Sporea I et al., [15] | Pradeep HN et al., [31] |
|---|
| F0-4.48±1.05 kPa | F0=<4.6 | F0=<7.1 kPa | <3.9 |
| F1-7.16±0.75 kPa | F1=4.6-5.6 kPa | F1=>7.1 kPa |
| F2-8.08±1.05 kPa | F2=5.7-7.0 kPa | F2=>7.8 kPa | <7.2 |
| F3-8.44±0.47 kPa | F3=7.1-12.0 kPa | F=>8 kPa | >7.2 |
| F4-16.01±3.78 kPa | F4=>12 kPa | F4=>11.5 kPa | >10 |
It has been observed that APRI values increased with increasing grades of fibrosis. As the liver inflammation increases, there is a significant increase in (ALT), (AST), and reduction of platelet counts because of reduced synthesis of factors required for platelet production and also from other coagulation abnormalities related to CLD [20]. Mean APRI values of the cases which were diagnosed as F1, F2, and F3 grades show significant overlap, hence it is convenient to include these into a single group [Table/Fig-9]. Further, these patients were categorised into several groups based on the grades of fibrosis; F0 vs F1-F4, F0-F1 vs F2-F4, F0-F2 vs F3-F4, F0-F3 vs F4, and comparison was done [Table/Fig-10]. The SWE cut-off value obtained for differentiating F0-F1 vs F2-F4 was more than 7.65 kPa with a sensitivity of 90%, specificity of 100% and AUROC of 0.96 [Table/Fig-11]. For F0-F2 vs F3-F4, the SWE cut-off value was more than 7.9 kPa with a sensitivity of 95%, specificity 96.5% and AUROC of 0.95. The accuracy of SWE for differentiating among various subgroups of fibrosis was comparable and even slightly higher than the previous study by Sande JA et al., [Table/Fig-10] [18].
Comparison of mean APRI values for corresponding histological grades of liver fibrosis obtained in the present study with previous studies [18].
| Study | Variables | F0 | F1 | F2 | F3 | F4 |
|---|
| Our study | Mean APRI values | 0.34±0.21 | 0.72±0.26 | 0.81±0.33 | 0.98±0.31 | 1.91±0.42 |
| Sande JA et al., [18] | Cut-off values | <0.5 | 0.5-1.5 | >1.5 | | |
Comparison of mean shear wave elastography among sub-groups of METAVIR system of the present study with previous study [18].
| Study | Variables | F0 VS F1-F4 | F0-1 VS F2-4 | F0-2 VS F3-F4 | F0-F3 VS F4 |
|---|
| Present study | Cut-off (kPa) | >6.5 | >7.65 | >7.9 | >10.85 |
| AUROC | 0.994 | 0.969 | 0.953 | 1 |
| 95% CI | 0.004 - 0.2 | 0.04 - 0.3 | 0.008 - 0.4 | - |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sande JA et al., [18] | Cut-off | - | - | - | - |
| AUROC | 0.879 | 0.908 | 0.929 | 0.926 |
| 95% CI | 1.79-4.025 | 1.839-4.838 | 1.432-2.236 | 1.379-2.115 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Comparison of Receiver operating characteristic curve analysis of SWE for different stages of fibrosis. SWE: Shear wave elastography; AUROC: Area under the receiver operating characteristic; CI: Confidence interval; kPa: Kilo-pascal
Sensitivity (89.7%) and specificity (100%) of Shear Wave Elastography in differentiating F0-F1 (No to mild fibrosis) from F2-F4 (clinically significant fibrosis) at cut-off value of >7.65 kPa.
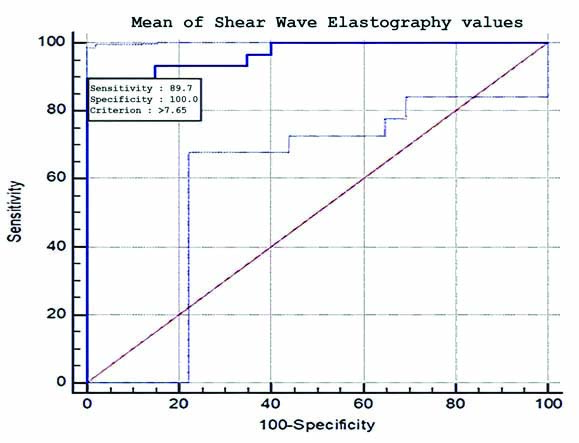
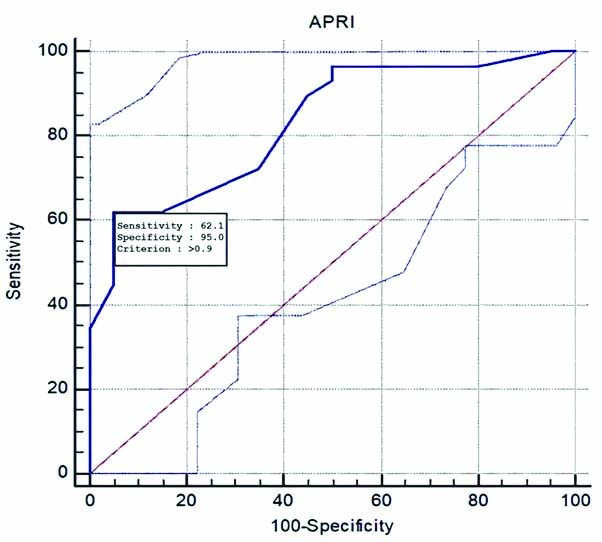
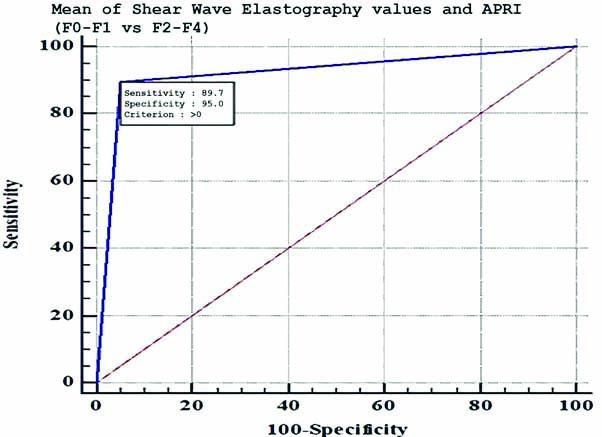
This study focuses on non significant fibrosis (F0-F1) vs significant fibrosis (F2-F4) of which the latter can be potentially reversed by antiviral therapy. There is a good correlation between SWE mean values and the histological fibrosis (METAVIR) scores. For differentiating F0-F1 from F2-F4 the best sensitivity (90%) matched with specificity (100%) was obtained at a mean SWE value of approximately 7.65 kPa, and for APRI the best sensitivity (62%) matched with specificity (95%) is at APRI value of 0.9 [Table/Fig-12,13] [18]. The AUROC for the SWE mean and APRI was 0.96 and 0.83, respectively. The SWE and APRI values showed a significant correlation with the histological fibrosis scores. The upper and lower limits of 95% confidence interval do not cross 1, further strengthening this result. However, the wide limits might have been due to the small sample size and since approximately 20% of the samples were of F0 grade. However, eliminating F0 grade would reduce the limits with a dubious effect on clinical significance. APRI together with SWE had an AUROC of 0.92, which suggests APRI does not have an added beneficial role in differentiating F0-F1 from F2-F4 when used along with SWE [Table/Fig-14,15] [18] this result is in line with the previous studies [18]. Although APRI values increase with increasing grade of fibrosis, it is less accurate than SWE in detecting and grading the fibrosis. The sensitivity and specificity of SWE in the detection of liver stiffness compared with biopsy results are in concordance with previous studies, however the sensitivity was slightly higher in the present study [Table/Fig-16] [15,31].
Comparison of mean APRI values among sub-groups of METAVIR system of the present study with previous study [18].
| Study | Variables | F0 VS F1-F4 | F0-1 VS F2-4 | F0-2 VS F3-F4 | F0-F3 VS F4 |
|---|
| Present study | Cut-off | >0.4 | >0.9 | >0.9 | >1.3 |
| AUROC | 0.925 | 0.83 | 0.864 | 0.997 |
| 95% CI | 1.2 - 45.3 | 1.8 - 85.6 | 2.1 - 14.0 | 5.8 - 277.1 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sande JA et al., [18] | Cut-off | - | - | - | - |
| AUROC | 0.803 | 0.780 | 0.784 | 0.812 |
| 95% CI | 1.89-11.41 | 1.72-7.01 | 1.01-1.93 | 1.06-2.14 |
| p-value | 0.0008 | 0.0005 | 0.039 | 0.0202 |
Comparison of Receiver operating characteristic curve analysis of APRI for different stages of fibrosis. APRI: Aminotransferase platelet ratio index, AUROC: Area under the receiver operating characteristic, CI: Confidence interval, kPa: Kilo-pascal
Sensitivity (62.1%) and specificity (95%) of APRI in differentiating F0-F1 (No to mild fibrosis) from F2-F4 (clinically significant fibrosis) at APRI value >0.9.
Comparison of mean SWE and APRI values among sub-groups of METAVIR system of the present study with previous study [18].
| Study | Variables | F0 vs F1-F4 | F0-1 vs F2-4 | F0-2 vs F3-F4 | F0-F3 vs F4 |
|---|
| Present study | AUROC of SWE and APRI | 0.925 | 0.923 | 0.889 | 0.988 |
| Sande JA et al., [18] | AUROC of SWE and APRI | 0.890 | 0.920 | 0.931 | 0.92 |
Comparison of Receiver operating characteristic curve analysis for SWE and APRI for different stages of fibrosis. SWE: Shear wave elastography; APRI: Aminotransferase platelet ratio index; AUROC: Area under the receiver operating characteristic; CI: Confidence interval; kPa: Kilo-pascal
Sensitivity (89.7%) and specificity (95%) of combined Shear Wave Elastography and APRI in differentiating F0-F1 (no fibrosis to mild fibrosis) from F2-F4 (clinically significant fibrosis).
The sensitivity and specificity of SWE for detection of mild liver fibrosis (F0-F1) [15,31].
| Study | Sensitivity (%) | Specificity (%) | AUROC |
|---|
| Present study | 90 | 100 | 0.96 |
| Pradeep HN et al., [31] | 75 | 100 | 0.98 |
| Sporea I et al., [15] | 76 | 82 | 0.82 |
AUROC: Area under the receiver operating characteristic
In our study, we found that SWE can accurately differentiate non significant from clinically significant fibrosis (F0-F1 from F2-F4) with a very high sensitivity (90%) and specificity (100%) at the mean cut-off value of >7.46 [Table/Fig-10]. APRI values significantly overlap among the F1-F3 group and we are unable to select the best cut-off value to differentiate F0-F1 from F2-F4 and F0-F2 from F3-F4 [Table/Fig-9]. Therefore, it does not appear to be useful in differentiating between various grades of liver fibrosis. Also, when used along with SWE, it does not significantly improve its diagnostic accuracy in liver fibrosis grading. Since, all the cases of F1-F3 grades fall in the APRI range of 0.5-1.5, a cut-off value of >1.5 can be used to differentiate F1-F3 (mild to severe fibrosis) from F4 (cirrhosis) with a very good sensitivity (100%) and specificity (97.5%) [Table/Fig-12]. The APRI being easily available and cost effective, can be a good non invasive serum marker to monitor the liver function in long term follow-up of patients with chronic progressive liver fibrosis.
The SWE is an easily available, repeatable and cost effective modality it can be used as a non invasive alternative to the biopsy for grading of liver fibrosis and follow-up of chronic viral hepatitis patients. Biopsy being a gold standard and invasive test, it can be reserved for specific clinical settings and for baseline evaluation of fibrosis. SWE has higher diagnostic accuracy compared to the APRI for grading as well as for differentiating non significant (F0-F1) from clinically significant (F2-F4) fibrosis. The APRI has a minimal role in the grading of the fibrosis, it can be combined with SWE for post-treatment follow-up of liver fibrosis patients along with other serological scoring systems thus avoiding the need for repeated biopsy.
Limitation(s)
There were some limitations in the study. SWE grading of fibrosis was done subjectively by a single radiologist. Hence, the inter-observer variation could not be evaluated. Since, the subjects were from a homogenous population of Indian adults, whether the results from this study can be extrapolated to other ethnic groups remain to be seen. Also, the large number of cases in housewives and health care workers might have been due to selection bias secondary to the spectrum of cases in our hospital. Most of the patients in our hospital are from the lower socio-economic strata, especially from rural regions. Larger clinical studies are recommended to validate the accuracy of SWE and APRI to establish threshold values for various grades of liver fibrosis in the Indian population as the majority of previous studies are done on the western population.
Conclusion(s)
The SWE has high accuracy in detecting and grading of fibrosis. SWE being a non invasive, easily available and repeatable test can be a promising alternative to the biopsy. It has higher diagnostic accuracy compared to the APRI for grading as well as for differentiating non significant (F0-F1) from clinically significant (F2-F4) fibrosis and helpful in planning appropriate anti-fibrotic or antiviral treatment. The APRI has a minimal role in the grading of the fibrosis, however it can be combined with SWE for post-treatment follow-up of chronic viral hepatitis patients thus avoiding the need for repeated biopsy.
@Comparision of mean values of liver elasticity value among age group of control with one-way ANOVA test; $Comparison of mean values of liver elasticity value among age group of case with one-way ANOVA test; #Comparision of mean values of liver elasticity value among cases and control are calculated with independent t-test.
Comparison of Receiver operating characteristic curve analysis of SWE for different stages of fibrosis. SWE: Shear wave elastography; AUROC: Area under the receiver operating characteristic; CI: Confidence interval; kPa: Kilo-pascal
Comparison of Receiver operating characteristic curve analysis of APRI for different stages of fibrosis. APRI: Aminotransferase platelet ratio index, AUROC: Area under the receiver operating characteristic, CI: Confidence interval, kPa: Kilo-pascal
Comparison of Receiver operating characteristic curve analysis for SWE and APRI for different stages of fibrosis. SWE: Shear wave elastography; APRI: Aminotransferase platelet ratio index; AUROC: Area under the receiver operating characteristic; CI: Confidence interval; kPa: Kilo-pascal
AUROC: Area under the receiver operating characteristic