Breast carcinoma predominantly affects women and needs alarming attention because it causes high morbidity and mortality. It is the most common cancer seen in females that affects 2.1 million women every year and also causes the maximum number of cancer-related deaths among women [1]. The spectrum of breast lesions which are grouped under Aberrations in the Normal Development and Involution of breast (ANDI) consists of benign lesions like fibroadenoma, fibrocystic disease and others such as phyllodes tumour, gynaecomastia and chronic mastitis. Malignant lesions include ductal carcinoma, lobular carcinoma, colloid carcinoma and medullary carcinoma [2]. Invasive Ductal Carcinoma No Special Type (IDC NST) was found to be the most common histological type (88%) followed by infiltrating lobular carcinoma (3.7%), colloid carcinoma (1.1%), ductal Carcinoma In Situ (DCIS) (1.1%), and metaplastic types (0.9%) [3].
It is important to recognise benign lesions to distinguish them from in situ and invasive breast cancer and to assess a patient’s risk of developing breast cancer, so that the most appropriate treatment modality for each case can be established [4]. Most of the breast lesions can be classified by well-defined morphologic criteria but cases with equivocal morphology require ancillary studies e.g., IHC, to reach accurate diagnosis [5].
Using a single or panel of marker proteins, the technique of IHC is used to characterise various tumour subtypes, to confirm parent tissue and to differentiate metastatic from primary tumour. Additionally, it provides useful information regarding prognosis, response to therapy or evaluating residual tumour after treatment. A wide range of antibodies are available which help in arriving at the final diagnosis in difficult cases using IHC and thus determine the prognosis and response to therapy in breast lesions [6]. Recently, p63, a p53 homologue, has been characterised as a reliable marker of myoepithelial cells of lactiferous duct. The p63 is exclusively expressed in myoepithelial cells of normal breast tissue and hence it can be of great help in differential diagnosis involving benign lesions. The p63 shows nuclear staining and lack of staining in smooth muscle cells like myofibroblasts and blood vessels makes it very advantageous to use [7].
Over the past decade, there has been significant progress in the discovery and application of antibody markers that can be used in the immunohistochemical identification of myoepithelial cells as they have proved to be of great utility in distinguishing benign from malignant lesion. Though all current markers remain imperfect due to their cross reactivities. But p63 is better choice due to its unique and near absolute specificity for breast tissue [8]. Hence, this study was planned to further elucidate whether there is any difference in expression of p63 among benign and malignant breast lesions or not.
Materials and Methods
This prospective study was carried over a period of one year from 1st December 2018 to 30th November 2019 in the Department of Pathology at Bhagat Phool Singh Government Medical College for Women, Khanpur Kalan, Sonepat, Haryana, India. The Institutional Ethical Committee clearance was taken for the study (BPSGMCW/RC375/IEC/18 dated 22/11/18).
Inclusion criteria: All breast trucut biopsy, incisional biopsy, lumpectomy and mastectomy specimens of patients with neoplastic etiology were included in the study. In the category of malignant lesions, only the histopathologically proven cases of breast carcinoma were included.
Exclusion criteria: All inflammatory/infective lesions were excluded.
Sample size calculation: The sample size was calculated using Master 2.0 software for two groups, benign and malignant, taking proportion 0.63 and 0.30 with estimated risk difference 0.33 at 95% confidence interval. The sample size was 76, out of which minimum size of 30 cases were included in each group.
Study Procedure
Tissues were routinely fixed in buffered formalin, grossed and processed.
Positive control for p63: A histological section of prostate biopsy was used as positive control with each batch of staining.
Negative control for p63: For negative control, flex negative control, mouse (code IR750) was used.
After proper grossing and processing of formalin fixed specimen, routine staining of sections using Haematoxylin and Eosin (H&E) stain was done. For IHC, from selected blocks 3-4 micron thick sections were taken on polylysine coated slides followed by deparaffinisation and hydration. Heat induced epitope retrieval using Tris/EDTA pH 9.0 buffer was done followed by endogenous peroxidase inactivation using aqueous hydrogen peroxide. Incubation with primary and secondary antibody followed by Diaminobenzediene (DAB) chromogen and counterstaining was carried out.
All histopathology and IHC stained slides of these cases were studied in details. Neoplastic lesions were categorised on the basis of World Health Organisation (WHO) classification of breast tumours 2019 [9]. Nottingham’s modification of Bloom-Richardson grading system has been used for histopathological grading [10].
Assessment of p63 expression: The intensity of p63 expression was evaluated as continuous positive, discontinuous positive and negative. The extent was scored on the basis of percentage of positive cells and assigned a score of negative (0%), 1 (<25%), 2 (26-90%) and 3 (91-100%) [8].
Statistical Analysis
Mean±SD and percentage has been used to determine statistical analysis for quantitative data. Chi-square test (Fischer’s-exact test) has been used. The results then obtained have been compared statistically by using software Statistical Package for the Social Sciences (SPSS) version 20 and p-value has been calculated. The p-value <0.05 has been considered statistically significant.
Results
A total of 76 breast specimens were included in study. Among 76 cases, there were 42 lumpectomy specimens (55.2%), 23 Modified Radical Mastectomy (MRM) specimen (30.3%), 10 trucut biopsy (13.2%) and one simple mastectomy specimen (1.3%). Out of total 76 cases, 38 cases (50%) were benign and 38 cases (50%) were malignant.
The patients included in the study were in the age groups of 14-75 years. Maximum number of cases were found in 5th decade followed by 4th decade of life. The mean age of presentation was 37.1 years. A total of 73 females and 03 males were included in present study. The most common histological variant of benign breast lesion was fibroadenoma followed by benign phyllodes tumour and borderline phyllodes tumour [Table/Fig-1]. Among the malignant lesions, the most common variant was infiltrating ductal carcinoma [Table/Fig-2]. Histological grading was done for 25 malignant cases. It was found that maximum number of cases i.e., 14 cases (i.e., 56%) were that of grade 2, followed by 06 cases (i.e. 24%) of grade 3, and 05 cases (i.e., 20%) of grade 1.
Histological diagnosis of various benign lesions (n=38).
| Diagnosis | No. of cases | Percentage (%) |
|---|
| Fibroadenoma | 20 | 52.63 |
| Benign phyllodes tumour | 09 | 23.68 |
| Borderline phyllodes tumour | 04 | 10.53 |
| Gynaecomastia | 02 | 5.26 |
| Adenomyoepithelioma | 01 | 2.63 |
| Sclerosing adenosis | 01 | 2.63 |
| Florid adenosis | 01 | 2.63 |
| Total | 38 | 100 |
Histological diagnosis of various malignant lesions (n=38).
| Diagnosis | No. of cases | Percentage (%) |
|---|
| Infiltrating ductal carcinoma | 34 | 89.47 |
| Ductal carcinoma in situ | 03 | 7.89 |
| Metaplastic carcinoma | 01 | 2.64 |
| Total | 38 | 100 |
Out of 38 malignant cases, lymph nodes were received for evaluation in 23 cases, in which MRM was performed. Out of which 14 cases (60.87%) showed lymph node metastasis while 09 cases (39.13%) were negative for lymph node metastasis. In lumpectomy cases and cases in which biopsy was done, lymph node status could not be assessed [Table/Fig-3]. The p63 immunostaining was performed in all cases. All the 38 benign cases showed positive expression (100%) of p63. Out of 38 malignant cases, 34 cases (89.5%) were devoid of p63 expression, while 04 cases (10.5%) showed positive p63 expression [Table/Fig-4]. Among 38 benign cases, 20 cases of fibroadenomas and 09 cases of benign phyllodes tumour show continuous p63 positivity with 3 scoring. Remaining 09 benign cases, showed discontinuous positivity [Table/Fig-5]. Out of 38 malignant cases, immunostaining for p63 were negative in 34 cases while remaining 04 cases show discontinuous positivity with p63 scoring 2 in metaplastic carcinoma (1 case) and score 1 in DCIS (3 cases) [Table/Fig-6].
Lymph node status in MRM cases (n=23).
| Lymph node metastasis | No. of cases | Percentage (%) |
|---|
| Present | 14 | 60.87 |
| Absent | 09 | 39.13 |
| Total | 23 | 100 |
p63 expression in benign and malignant cases (n=76).
| Category | Positivity for p63 | Negativity for p63 | Total |
|---|
| No. | % | No. | % | No. | % |
|---|
| Benign | 38 | 100 | 00 | 00 | 38 | 50 |
| Malignant | 04 | 10.5 | 34 | 89.5 | 38 | 50 |
| Total | 42 | 55.3 | 34 | 44.7 | 76 | 100 |
p63 expression and scoring in various histopathological types of benign lesions (n=38).
| Diagnosis | No. of cases | p63 expression | p63 score |
|---|
| Fibroadenoma | 20 | Continuous positive | 3 |
| Benign phyllodes tumour | 09 | Continuous positive | 3 |
| Borderline phyllodes tumour | 04 | Discontinuous positive | 2 |
| Gynaecomastia | 02 | Discontinuous positive | 2 |
| Adenomyoepithelioma | 01 | Discontinuous positive | 2 |
| Sclerosing adenosis | 01 | Discontinuous positive | 2 |
| Florid adenosis | 01 | Discontinuous positive | 2 |
| Total | 38 | | |
p63 expression and scoring in various histological types of malignant lesions (n=38).
| Diagnosis | No. of cases | P63 expression | p63 score |
|---|
| Metaplastic carcinoma | 01 | Discontinuous positive | 2 |
| Ductal carcinoma in situ | 03 | Discontinuous positive | 1 |
| Infiltrating ductal carcinoma | 34 | Negative | 0 |
| Total | 38 | | |
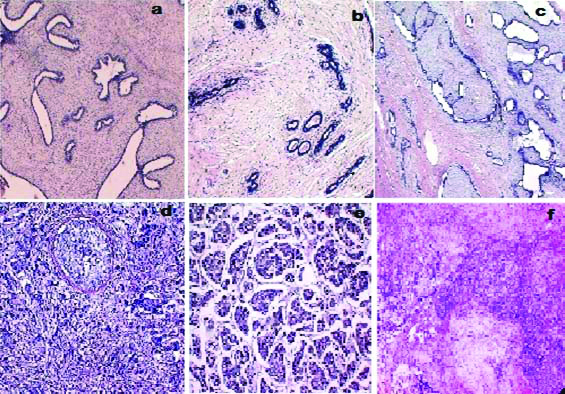
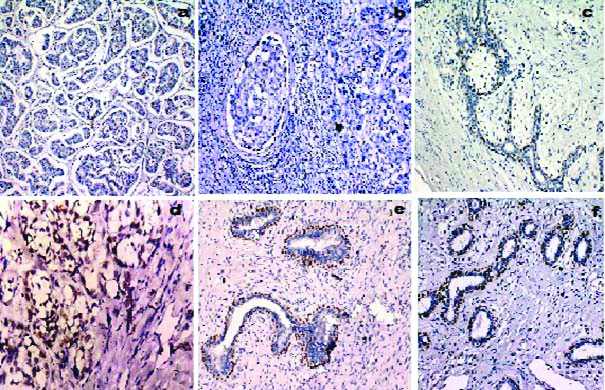
Haematoxylin and Eosin stained sections of various benign and malignant lesions of breast included in the study like fibroadenoma, borderline phyllodes tumour, benign phyllodes tumour, DCIS, invasive ductal carcinoma and metaplastic carcinoma shown in [Table/Fig-7]. IHC expression of p63 sections of various benign and malignant lesions of breast included in the study like invasive ductal carcinoma, DCIS, borderline phyllodes tumour, metaplastic carcinoma and fibroadenoma shown in [Table/Fig-8].
a) Fibroadenoma showing biphasic proliferation of duct and stroma with pericanicular stromal growth pattern (H&E, 40X); b) Borderline phyllodes tumour showing increased stromal cellularity. (H&E, 100X); c) Benign phyllodes tumour showing leaf like architecture (H&E, 40X); d) Ductal carcinoma in situ showing neoplastic cells within the duct. (H&E, 100X); e) Invasive ductal carcinoma showing nests and island of tumour cells. (H&E, 100X); f) Metaplastic carcinoma showing chondroid differentiation of tumour cells with area of epithelial differentiation (H&E, 40X).
a) Invasive ductal carcinoma showing negative immunostaining for p63, score 0 (p63, 100X); b) Ductal carcinoma in situ showing discontinuous immunostaining for p63, score 1 (p63, 100X); c) Borderline phyllodes tumour showing immunostaining for p63, score 2 (p63, 200X); d) Metaplastic carcinoma showing immunostaining for p63, score 2 (p63, 100X); e) Fibroadenoma showing immunostaining for p63, score 3. (p63, 200X); f) Fibroadenoma showing immunostaining for p63, score 3 (p63, 200X).
On comparing the expression of p63 in benign and malignant breast lesions a significant difference was found between the two categories. The p63 was found to be consistently positive in benign lesions [Table/Fig-9]. This signifies that IHC expression of p63 can be used as reliable marker to distinguish between benign and malignant breast lesions.
Association between p63 in benign and malignant lesions.
| p63 expression in benign and malignant cases (n=76) |
|---|
| Category | Positivity for p63 n (%) | Negativity for p63 n (%) | p-value |
|---|
| Malignant | 4 (10.5) | 34 (89.5) | 0.001 |
| Benign | 38 (100.0) | 0 |
A p-value <0.001 (p<0.05) was calculated i.e., statistically highly significant. (Fischer-exact test)
Discussion
Myoepithelial cells in normal breast express p63 exclusively and hence p63 can be very useful in differential diagnosis involving benign lesions. The p63 shows nuclear staining and absence of staining in smooth muscle cells, such as myofibroblasts and blood vessels thus making it very advantageous [7]. Hence, specificity of p63 is approximately 100% however, its sensitivity has been found to be approximately 90% [7]. In the previous studies, the myoepithelial cell layer surrounding the luminal epithelial cells showed p63 immunoreactivity in all benign breast lesions. Whereas in carcinoma in situ, a less continuous peripheral rim of myoepithelial cells was spotted with p63-stain. All invasive breast carcinomas lacked peripheral p63 staining [11].
Based on histopathological features, 50% cases of our study were found to be benign and 50% cases were malignant. Our findings were similar to Thakkar B et al., and Bukhari MH et al., who reported 54.16% and 60% benign cases respectively in their studies [12,13]. In the study by Modi P et al., 72% benign and 16.7% malignant cases were found [14]. Chaudhary S et al., and Rahman MZ and Islam S, reported 19.23% and 14.7% malignant cases respectively [15,16].
In the benign lesions, fibroadenoma accounted for the maximum cases i.e., 52.63% in our study. This finding was consistent with most of the available literature on benign breast lumps, where fibroadenoma accounted for 46.6%-55.6% of all cases [17,18]. Invasive ductal carcinoma was the most common malignant lesion in our study constituting about 89.47% of all the malignant cases. This finding bears similarity with the studies of Bhagat VM et al., (94.82%), Juneja S et al., (92.72%), Verma N et al., (87.5%) and Tiwari N et al., (84.3%) [19-22]. One case of metaplastic carcinoma was found in our study. Metaplastic Breast Carcinoma (MBC) is a rare heterogeneous group of primary breast malignancies accounting for less than 1% of all invasive mammary carcinomas. These tumours express epithelial and/or mesenchymal differentiation within the same tumour [23].
In our study, 100% of benign tumours were positive for p63. Majority i.e., 34 cases (89.5%) of malignant tumours were devoid of p63 positivity, however, four cases i.e., 10.5% of malignant lesions expressed p63. These four cases included three cases of DCIS and one of metaplastic carcinoma. Results similar to our study were reported by Barbareschi M et al., who found p63 positivity in all benign lesions while invasive breast carcinomas were consistently devoid of nuclear p63 staining in (95%) [24]. Similarly, Wang X et al., investigated benign and malignant lesions of breast for p63 expression and found that p63 was exclusively expressed in the myoepithelial cells of normal breast, partially expressed in ductal hyperplasia, rarely expressed in carcinoma in situ and not at all expressed in invasive carcinomas [25]. In the study conducted by Stefanou D et al., nuclear immunoreactivity of p63 was seen in benign breast lesions in all cases [11]. In malignant lesions only 11.9% cases showed staining for p63. A comparative study conducted by Yanping D and Qiurong R revealed that all benign lesions were positive for p63 [26]. In malignant lesions, 26 cases including 19 cases of DCIS and 7 cases of intraductal papillary carcinoma were positive for p63 and remaining 19 IDC cases were negative for p63. Verma N et al., conducted study on total of 151 cases and found that the expression of p63 in malignant cases showed 100% negativity and in benign cases 88.6% showed positivity and 11.4% were negative [21]. Further in benign category, non proliferative lesions revealed continuous positive pattern for p63 while proliferative lesions showed discontinuous positivity for p63. In a study done by Ranjan R et al., on 98 cases, revealed 100% positivity for p63 in benign cases while malignant cases show only 17.6% positivity for p63 [27].
The results of our study differ greatly from Harton AM et al., they studied p63 expression in breast lesions and reported that out of 29 benign lesions, 25 showed 75% or more p63 staining in epithelial cell clusters whereas, in 11 out of 17 malignant lesions showed no staining for p63 in any of the epithelial cell clusters. The DCIS showed 75% positivity for p63 in the cell clusters, and the infiltrating ductal carcinoma showed 60% [28].
On comparing the expression of p63 in benign and malignant breast lesions using Fischer’s-exact test, a significant difference (p-value <0.001) was found between the two categories. Hence, our study concludes that p63 can be used as a marker to differentiate between benign and malignant breast lesions.
Limitation(s)
The mild variation in the results of present study from previous literature can be attributed to the small sample size. Also, malignant lesions of different histological subtypes need to be evaluated for p63 expression which was lacking in the present study.
Conclusion(s)
The IHC has become a standard tool in diagnosing, deciding therapy and prognosis for carcinoma breast. As per previously reported studies, p63 has been found to be a very useful IHC marker in cases that are difficult to identify on the basis of histomorphological features alone, borderline cases and cases of carcinoma in situ. The present study was done to correlate IHC expression of p63 in benign and malignant breast lesions. Our study concluded that there is a definite difference in p63 staining between benign lesions and in situ carcinomas on one hand and invasive carcinomas on the other. In the present study, p63 was found consistently positive in benign lesions while in malignant cases, consistent absence of p63 expression was noted. Hence, p63 expression is useful for diagnosis in trucut biopsy specimen preoperatively and excisional lumpectomy specimen postoperatively.
A p-value <0.001 (p<0.05) was calculated i.e., statistically highly significant. (Fischer-exact test)