The World Health Organisation (WHO) defined stroke as “rapidly developing clinical signs of focal or global disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than vascular origin” [1]. Acute ischaemic stroke is caused by sudden interruption of cerebral blood flow. The causes of acute ischaemic stroke in most patients who have severe symptoms include embolic or thrombotic occlusion (70-80% of patients) [2]. Multiple studies reveal that inflammation has a significant role in the pathogenesis of atherosclerotic stroke through mechanisms like increasing the serum levels of cytokines, fibrinogen, clotting factors, and leukocytes by altering the functions of endothelial cells.
Systemic inflammatory response occurs after ischaemic events and is responsible for thrombosis progression. Several studies have indicated that higher levels of inflammatory biomarkers such as CRP and Interlukin-6 (IL-6) have been associated with worsening of ischaemic events [3]. The Framingham study showed that CRP is an important predictor of ischaemic stroke and Transient Ischaemic Attack (TIA) [4]. Also, raised baseline high sensitivity-CRP (hs-CRP) levels are independently associated with excessive ischaemic stroke risk but exhibit no clear effect on haemorrhagic stroke [5].
Multiple studies have demonstrated that CRP concentrations are predictive of future cardiovascular events in stroke patients and also CRP values are strongly correlated with stroke severity and disability [15-19]. Hence, elevated CRP is a predictive marker for future cardiovascular events, but the timing of CRP evaluation in relation to the qualifying event remains undetermined. With this backdrop of knowledge, in this study, authors intended to evaluate the prognostic significance of CRP as an inflammatory marker in acute cerebral ischaemic stroke. The aim of the present study was to assess the significance of CRP values in prognosis and outcome of patients having acute ischaemic stroke.
Materials and Methods
The present study was a prospective single centre observational study which was conducted on a total number of 62 patients with clinical and CT brain diagnosed first ischaemic stroke admitted to SCB Medical College and Hospitals, Cuttack, Odisha, India, from May 2017 to August 2019. The study protocol was approved by Institutional Ethical Committee, SCB Medical College, Cuttack, Odisha, India (reference number 121/4.4.2017).
Sample size calculation: The sample size was calculated to be 54 (by Andrew Fisher formula using 95% confidence level, a standard deviation of 0.5 and a confidence interval of ±5%). The final sample size taken for the study was 62.
Inclusion criteria: All patients of clinical and CT scan brain confirmed first ischaemic stroke admitted within 72 hours of symptom onset were included in the study. Consent was obtained from patient or from guardian if patient was unable to give consent.
Exclusion criteria: Patients having acute infectious disease, stable or unstable angina, acute myocardial infarction, immunological disorders, known or suspected neoplastic disorders, recent history of (<3 months) major trauma, surgery, burns, osteoarthrosis, costochondritis, rheumatoid arthritis, ankylosing spondylitis, renal failure, haemorrhagic stroke, collagen vascular disease, liver disease, etc were excluded from the study.
Medical history was taken from the patient or his/her relatives if the patient was unconscious or not able to speak. Physical examination was performed by neurology residents. Patients were evaluated for age, sex, diabetes, hyperlipidaemia, ischaemic heart diseases, smoking and past history of stroke or hypertension. Routine laboratory tests, brain Magnetic Resonance Imaging (MRI), Transthoracic Echocardiography (TTE) and carotid doppler ultrasonography were done in all the patients.
Study Protocol
At admission, initial stroke severity and disability were assessed by the Canadian Neurological Stroke Scale (CNSS) and Barthel Index (BI), respectively [20,21]. Besides other routine laboratory investigations, CRP level in patients of acute ischaemic stroke was estimated quantitatively at the time of admission and discharge. The patients were followed-up at one month, three months and six months. During each follow-up visit, the disability score was calculated according to BI.
CRP value was correlated with infarct size (detected by CT scan). The CRP was correlated with mortality and morbidity (disability, vascular events). The patients were regularly followed-up over phone every weekly intervals to know about mortality and morbidity. The end events were death or any new non fatal vascular events (recurrent stroke, unstable angina, myocardial infarction whichever came first) recorded during the six months follow-up period.
The patients were divided into three groups based on CRP values i.e., Low CRP<5 mg/L, Medium=5-33 mg/L, and High CRP >33 mg/L as done in previous studies [22] (Di Napoli M et al., 2001).
C-Reactive Protein Assay
All the blood samples were collected from the patients 1st at time of admission and then at discharge and hs-CRP was measured with an immunoturbidimetry assay on an Architect c16000 chemistry analyser (Abbott Diagnostics, Abbott Park, USA). The results were expressed in mg/L. Normal reference of CRP was less than 5 mg/L.
CT Scan of Brain
For each patient CT was done at the time of admission in the Department of Radiodiagnosis, Regional Diagnostic Centre, SCB Medical College, Cuttack. CT scan was used to differentiate between haemorrhagic and ischaemic stroke. The American Heart Association (AHA) and American Stroke Association (ASA) emphasise on need of an urgent brain imaging study prior to administration of stroke-specific treatment and a non enhanced CT scan supplies enough information for clinical decision-making in this setting. Wider availability and faster acquisition time as compared to MRI make the CT scan a more practical option and hence, worldwide, it is the most commonly used diagnostic imaging test used in acute ischaemic stroke. Additional time is consumed in MRI in order to screen for contraindications and proper positioning of the patient on the table. Moreover with technological advancement, ultra rapid CT scan are equally efficient as MRI in revealing ischaemic changes and viable brain parenchyma. On account of the above factors, CT scan was preferred over MRI in present study.
Statistical Analysis
The data were analysed using SPSS version 11.0 for statistical analysis. The statistical procedures include proportions, Chi-square test for association, Analysis of Variance (ANOVA) for testing the difference between means and logistic regression for estimating the Relative Risk (RR).
Results
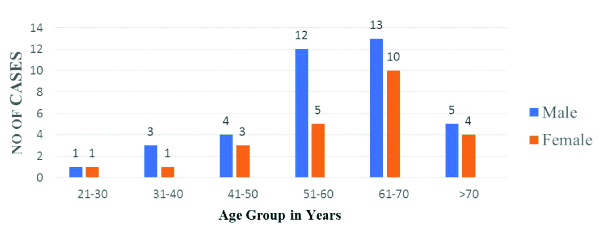
The baseline characteristics of the patient population is shown in [Table/Fig-1]. In the present study, maximum number of patients was found in age group 61-70 years (37%). Incidence among male predominates over female [Table/Fig-2]. Out of all risk factors, hypertension amounts to be the maximum i.e., 67.7% cases and incidence was highest at medium value of CRP 5-33 [Table/Fig-3] followed by dyslipidaemia. Out of all cardiovascular features, left ventricular hypertrophy amounts to be the highest i.e., 45.1% which occurs maximum at high CRP level group i.e., >33 [Table/Fig-4].
| Characteristic | Number (%) |
|---|
| Mean age (years) | 56.3 |
| Male gender | 38 (61.2) |
| Hypertension | 42 (67.7) |
| Diabetes | 18 (29) |
| Dyslipidemia | 26 (42) |
| Chronic heart disease | 12 (19.3) |
| Smoking | 11 (17.7) |
| Previous ischaemic stroke | 8 (12) |
| History of antiplatelet use | 10 (16) |
Age and Sex distribution of ischaemic stroke.
Distribution of risk factors in relation to level of CRP at admission.
| Risk factors | Total (n=62) | CRP value (mg/L) |
|---|
| <5 (n=16) | 5-33 (n=34) | >33 (n=12) | p-value (Chi-square test) |
|---|
| Hypertension | 42 (67.7) | 10 (63) | 23 (68) | 9 (75) | 0.78 |
| Diabetes | 18 (29) | 7 (39) | 9 (27) | 3 (25) | 0.41 |
| Dyslipidaemia | 26 (42) | 5 (31) | 15 (35) | 6 (18) | 0.56 |
| CHD | 12 (19.3) | 2 (13) | 6 (18) | 4 (33) | 0.35 |
| Smoking | 11 (17.7) | 3 (19) | 5 (15) | 3 (25) | 0.71 |
| No risk factors | 4 (6.5) | 1 (6) | 2 (6) | 1 (6) | 0.95 |
Comparision of cardiovascular features in relation to CRP value at admission.
| Clinical characteristics | Total (N=62) | CRP value (mg/L) | p-value (Chi-square test) |
|---|
| <5 (n=16) | 5-33 (n=34) | >33 (n=12) |
|---|
| Atrial fibrillation | 4 (6.5) | 1 (6.3) | 3 (8.8) | 0 | 0.56 |
| Peripheral arterial disease | 6 (9.7) | 1 (6.3) | 3 (8.8) | 2 (16.7) | 0.63 |
| Left ventricular hypertrophy | 28 (45.1) | 6 (37.5) | 16 (47) | 6 (50) | 0.76 |
| Carotid stenosis | 5 (8) | 1 (6.3) | 3 (8.8) | 1 (8.3) | 0.38 |
The severity of stroke, extent of disability and infarct size were all significantly higher in the high CRP group [Table/Fig-5]. In high CRP group out of 12 patients, 4 (33.3%) patients suffered fatal events and 4 (33.3%) suffered non fatal events. So, 8 patients (66.6%) suffered adverse events, which was statistically significant compared to low and medium CRP group [Table/Fig-6]. Out of all the total number of events, maximum occurred in medium CRP level group i.e., 35.3%, but the RR was highest in CRP level >33 i.e., 6.1 which is significant [Table/Fig-7].
Comparision of neuroradiological features with CRP at time of admission.
| Neuroradiological features | CRP value (mg/L) | p-value (Chi-square test) |
|---|
| <5 (n=16) | 5-33 (n=34) | >33 (n=12) |
|---|
| CNSS (mean) | 7 | 6.1 | 5.3 | 0.023 |
| Barthel Index (mean) | 36.8 | 30.3 | 30 | 0.016 |
| Infarct size in mm (mean) | 2.9 | 4.3 | 6 | 0.012 |
p-value <0.05 considered significant
Comparison of CRP at admission with end points at 6 month.
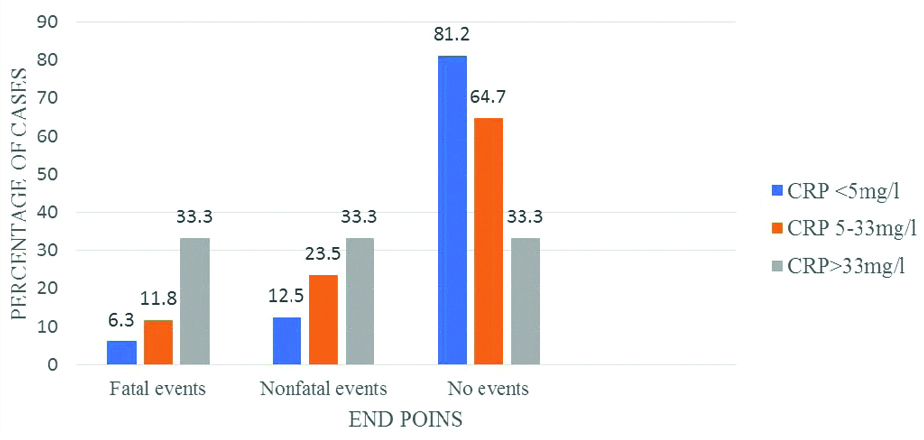
Comparison of CRP at admission and Relative Risk (RR) of end points at 6 months.
| CRP (mg/L) | Event | RR | p-value (Chi-square test) |
|---|
| <5 | 3 (18.8%) | 1 | - |
| 5-33 | 12 (35.3%) | 2.7 | 0.17 |
| >33 | 8 (66.7%) | 6.1 | 0.03 |
p-value <0.05 considered significant
A 80% of patients in the high CRP group suffered fatal and non fatal events which was statistically significant [Table/Fig-8]. A high CRP at discharge is associated with 19.4 times higher risk of adverse events which is statistically significant [Table/Fig-9]. Out of 14 non fatal events, four belonged to high CRP range, 8 belonged to medium CRP and two belonged to low CRP group [Table/Fig-10]. BI at admission and 6 months are both lowest in the high CRP at admission group. These values are statistically significant [Table/Fig-11]. BI at admission and six months were lowest in the high CRP at discharge group which are highly significant [Table/Fig-12]. So BI at admission more strongly correlated with CRP at discharge (p=0.008) than CRP at admission (p=0.016). Also, BI at six month more strongly correlated with CRP at discharge (p=0.001) than CRP at admission (p=0.01).
Comparison of CRP at discharge with end points at 6 month.
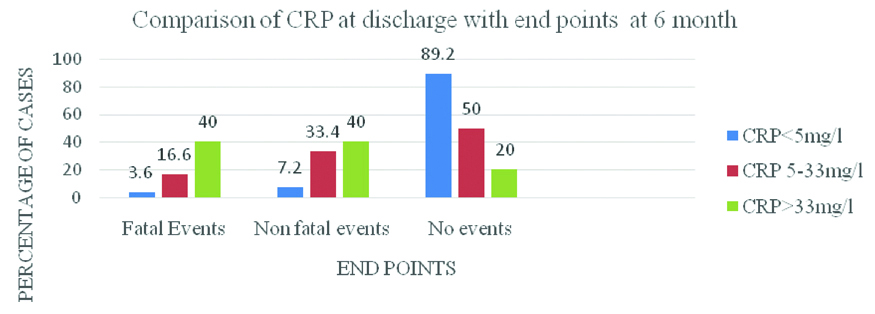
Comparision of CRP at discharge and Relative Risk (RR) of end points at 6 months.
| CRP (mg/L) | Event | RR | p-value (Chi-square test) |
|---|
| <5 | 3 (10.8%) | 1 | - |
| 5-33 | 12 (50%) | 9.8 | 0.002 |
| >33 | 8 (80%) | 19.4 | 0.001 |
p-value <0.05 considered significant
Distribution of non fatal events in relation to CRP values.
| Events | Total (N=62) | CRP value (mg/L) |
|---|
| <5 | 5-33 | >33 |
|---|
| Restroke | 7 | 1 | 4 | 2 |
| Unstable angina | 5 | 1 | 3 | 1 |
| AMI | 2 | 0 | 1 | 1 |
| Total | 14 | 2 | 8 | 4 |
Comparison of disability (Barthel Index) with CRP at admission.
| Barthel index | CRP value (mg/L) | p-value (Chi-square test) |
|---|
| <5 (n=16) | 5-33 (n=34) | >33 (n=12) |
|---|
| At admission (Mean) | 36.8 | 30.3 | 30 | 0.016 |
| At 6 months (mean) | 72.5 | 62.6 | 51.2 | 0.01 |
p-value <0.05 considered significant
Comparision of disability (Barthel Index) with CRP at discharge.
| Barthel index | CRP values (mg/L) | p-value (Chi-square test) |
|---|
| <5 (n=28) | 5-33 (n=24) | >33 (n=10) |
|---|
| At admission (Mean) | 36.8 | 27.3 | 26.5 | 0.008 |
| At 6 months (mean) | 75.4 | 52.3 | 41.6 | 0.001 |
p-value <0.05 considered significant
Discussion
In the present study, out of 62 patients in the third to more than seventh decade, maximum number of cases was found in 61-70 years (37%) [Table/Fig-1]. This was in accordance with studies that state that the most common age group for ischaemic stroke is 60-69 years [22]. Patients more than 60 years age comprised 51.6% of total cases. This coincides with earlier studies wherein stroke was more common in elderly population [23]. In the present study, male predominates over female 38 (61.2%) in male and 24 (38.8%) in female which corroborates with Bejot Y et al., [24]. Among the risk factors [Table/Fig-3], the most common risk factor was hypertension 42 (67.7%) followed by dyslipidaemia 26 (42%) and diabetes in 18 (29%). This coincides with study done by Di Napoli M et al., Winbeck et al,. For every risk factor, the patients were divided according to CRP level into three groups. On statistical analysis, it was observed that there was no significant difference between the incidence of risk factor in the three groups of different CRP values, as evidenced by all p values calculated to be more than 0.05. So CRP is a considered as an independent factor and is not influenced by the presence of the aforesaid risk factors as seen in the present study and also in studies conducted earlier [25].
In present study [Table/Fig-4], incidence of atrial fibrillation (6.5%), peripheral arterial disease (9.7%), Left ventricular hypertrophy (45.1%) and carotid stenosis in 5 (8%) which was found to be not significantly different among the three group. The severity of stroke was assessed by CNSS score [Table/Fig-5] and it was observed that the mean CNSS score was 7 in the low CRP group, 6.1 in medium CRP group, and 5.3 in high CRP group. The difference in values are statistically significant (p=0.023). The mean BI score in the low CRP group is 36.8, medium CRP group is 30.3 and in high CRP group is 30 (p=0.016). The mean infarct size in low CRP group is 2.9 mm, in medium CRP group 4.3 mm and in high CRP group 6.0 mm (p=0.012). So the severity of stroke, disability and infarct size increases significantly with increase in CRP. The findings corroborate with that of Shoaeb MA et al., which states that serum CRP level on admission can be used to predict severity and early outcome in ischaemic stroke [27]. Authors found that the serum CRP level, measured within 24 hour of stroke onset, was significantly correlated with disease severity and outcome in ischaemic stroke.
Many investigators have found a wide range of increase in CRP after stroke [27,28]. Di Napoli M et al., found that CRP concentration increased in the first 24 hour following stroke; this increment was associated with the size of the infarction, so mounting CRP levels in the first 24 hour were synchronised to poor prognosis [29]. The association between high CRP and a high stroke severity remains unexplained. Atherothrombosis of the cerebral vessels is considered an inflammatory disorder with acute phase reactant proteins produced in the first few hours. The degree of inflammation determined by elevated CRP levels has been associated with an increased risk of vascular complications [30]. There is a distinct possibility that elevated CRP is a direct response to the extent of cerebral tissue injury [30].
As an inflammatory marker, it is possible that high CRP is associated with underlying processes that cause more severe strokes. Another link is the activation of coagulation by the elevated CRP levels through the important role of tissue factor expression. Previous data showed that activation of coagulation factors in stroke patients increased mortality, and fibrinogen has a putative role [31]. In the present study, during the follow-up period of six months, the fatal and non fatal outcomes were compared with CRP values at admission [Table/Fig-9]. Maximum percentage of events occurred in high CRP groups (66.7%). There was a statistical significance difference in the occurence of events in the 3 groups.(p=0.03). Taking the RR of low CRP as 1, the RR of an event in medium CRP group is 2.7 (p=0.17), which is not significant and RR of high CRP group is 6.1 (p=0.03), which was significant [Table/Fig-7].
The fatal and non fatal end points were also compared with the CRP values at discharge [Table/Fig-12]. Out of 23 cases, 3 case (10.8%) belonged to low CRP group, 12 (50%) to medium CRP group and 8 (40%) to the high CRP group. The difference in values was highly statistically significant (p=0.007). The highest percentage of death also occurred in high CRP group. There was a significant difference of incidence of deaths in among three CRP groups (p=0.01). Also, highest percentage of non fatal events occurred in high CRP group. So there was a significant difference in the incidence of non fatal events in the 3 groups. (p=0.01). The RR score of the end point was 1 in low CRP group, 9.8 in medium CRP group (p=0.002) and 19.4 in high CRP group (p=0.001) which was highly significant [32] [Table/Fig-9]. Hence, CRP at admission correlates with occurrence of fatal events only but CRP at discharge correlates with the occurrence of both fatal and non fatal events on follow-up. Also, CRP at discharge correlates more strongly with occurrence of events than CRP at admission.
Hence CRP at hospital discharge (p=0.002 and p=0.001) is a stronger predictor of events at 6 months than CRP at admission (p=0.17 and p=0.03) [33]. [Table/Fig-10] revealed that the most common adverse event on follow-up was restroke followed by unstable angina and myocardial infarction. The ASIST study revealed that among a battery of biomarkers, only hs-CRP is a predictor of further cerebrovascular events with an odds ratio of 1.14. The highest number of adverse events was seen in the middle CRP group [34]. In the present study [Table/Fig-11,12], the mean BI at admission is more strongly correlated with CRP at discharge ((p=0.008) than CRP at admission (p=0.016). Also, BI at 6 months is more strongly correlated with CRP at discharge (p=0.001) than CRP at admission (p=0.01). The present study also reveals that patients with elevated CRP had a significantly lower follow-up BI. The protective effect of CRP apheresis on severity of ischaemic stroke is being studied in the CASTRO1 study, the results of which are expected in 2022 [35, 36].
Limitation(s)
The present study was a small, single centre study, the results of which need to be validated in a larger study. Correlation of infarct size on CT scan and CRP would confirm direct pathophysiological basis of rise in CRP. Also, effect of treatment on long term prognosis (e.g., single vs dual antiplatelet) would elucidate practical application of measurement of CRP.
Conclusion(s)
Present study showed that CRP, a marker of inflammation rises significantly in patients of ischaemic stroke. The degree of rise of CRP indicates the severity of stroke as well as adverse outcomes. CRP at discharge is a better outcome in terms of fatal and non fatal events than CRP at admission. CRP also correlates with the magnitude of disability. All patients found to be at high risk were followed-up more frequently and meticulously. More emphasis was placed on lifestyle modifications including cessation of smoking, decreased alcohol use and increased physical activity. Short term dual antiplatelet therapy was given for 21 days followed by single antiplatelet as is recommended by the 2019 AHA/ASA guidelines in high risk cases.
p-value <0.05 considered significant
p-value <0.05 considered significant
p-value <0.05 considered significant
p-value <0.05 considered significant
p-value <0.05 considered significant