Gastric adenocarcinoma is the fifth most common malignancy diagnosed in the world according to GLOBOCON 2020 data. The incidence of gastric cancer is two-fold greater in men than in women [1]. The HER2 overexpression in gastric adenocarcinoma was first described in 1986 [2]. Overexpression of HER2 on tissue sections is defined as strong positive staining with IHC (3+ staining). In cases expressing only moderate expression (2+) on IHC, in situ hybridisation is used to confirm the HER2 status. The overexpression of HER2 in gastric adenocarcinoma has been reported in the range of 7-44% [3]. In the Indian population, the overexpression of HER2 has been reported to vary from 21-44% [4]. Trastuzumab is used as targeted therapy against HER2 in patients who showed HER2 gene overexpression by IHC or by in situ hybridisation with significant positive effects on the outcome [5].
The HER2 testing is often performed on endoscopic biopsies, but these may not always be representative of the HER2 expression of the whole tumour, especially since gastric adenocarcinomas are known to show intra-tumoural heterogeneity for HER2 overexpression [6]. Intratumoural heterogeneity, could potentially lead to false negative results and undertreatment of patients who could benefit from trastuzumab therapy [5,7]. The present study compared the HER2 expression in endoscopic biopsies and matched resection specimens of patients with gastric adenocarcinoma. To study the association of HER2 overexpression with Lauren classification and histologic grading.
Materials and Methods
The study was a prospective observational study, approved by the Institutional Review Board (IRB Min no.10206 dated 08/08/2016).
Inclusion criteria: Gastric mucosal biopsies and matching resection specimens for adenocarcinomas, received in the Department of Pathology, Christian Medical College, Vellore, Tamil Nadu, India, for a period of one year (1st July 2016 to 30th June 2017) were included in this study.
Exclusion criteria: The tumours of the Gastro-esophageal Junction (GEJ) were excluded.
The Haematoxylin and Eosin (H&E) slides were reviewed and classified by Lauren’s [8] and American Joint Committee on Cancer (AJCC) histologic grading (8th edition-2017) [9]. The histologic grading was defined by the degree of glandular formation (>95% for well differentiated, 50-95% for moderate and <50% for poor). Lauren classification distinguishes between intestinal type with the presence of gland formations, and diffuse type, with a pattern of poorly cohesive cells, high invasiveness, and the presence of signet ring cells. The demographic data was collected from the electronic database of the hospital.
One representative block from each resection specimen was chosen and a fresh section from the paraffin block was stained for HER2 using automated slide stainer, Ventana Bench mark XT. The HER2 IHC was scored using gastric HER2 criteria [6,10]. An IHC score of 3+ was considered as positive, 2+ was equivocal and 1+ and 0 were negative. Scores were assigned as follows: no positive staining or staining of only a part of cell membrane in less than 10% of cells (0, negative); barely visible staining of only a part of cell membrane in at least 10% of cells (1+, negative); a weak-to-moderate, complete, or basolateral positive staining in at least 10% of cells (2+, equivocal); and a moderate-to-strong, complete, or basolateral positive staining in at least 10% of cells (3+, positive). In biopsies, a cluster of at least five positive tumour cells was required to qualify as 3+. Discordance was defined as a state in which the score in the biopsy was negative but positive in the matched resection specimen or vice versa [11]. THe HER2 overexpression heterogeneity was defined as 10-90% of the tumour cells showing 3+ positive areas [12].
Statistical Analysis
The statistical analysis was done using SPSS software (version 25) and chi-square test was done for statistical significance (p<0.05).
Results
A total of 72 patients were identified with paired biopsy and resection specimens. The mean age was 53.1 (23-82 years) with a male preponderance (n=49; 68.1%). Majority of the cases belonged to the poorly differentiated grade (n=42; 58.3%) and diffuse subtype of Laurens classification (n=44; 61.1%). The overall HER2 positivity rate was 11.1% (8/72).
The HER2 positive rates (score 3+) were 9.72% on biopsy (7/72) and 8.33% on resection (6/72) [Table/Fig-1]. Fourteen of 72 patients (19.4%) had received NACT. The concordance rate for HER2 between biopsies and resection was 95.83%.
Overall IHC staining categories for HER2.
| Biopsy | Resection | Total |
|---|
| IHC | IHC | IHC | IHC |
|---|
| 0 | 1+ | 2+ | 3+ |
|---|
| IHC 0 | 60 | 1 | 0 | 1 | 62 |
| IHC 1+ | 0 | 3 | 0 | 0 | 3 |
| IHC 2+ | 0 | 0 | 0 | 0 | 0 |
| IHC 3+ | 2 | 0 | 0 | 5 | 7 |
| TOTAL | 62 | 4 | 0 | 6 | 72 |
In 60 cases, both biopsies and resection showed HER2 score 0, three mucosal biopsies and resection showed HER2 score 1+ and five mucosal biopsies and resection showed HER2 score 3+. None of the cases showed HER2 score 2+. In one case, the HER2 staining was 0 on biopsy and was 1+ on resection and one case was 0 on biopsy and 3+ on resection. Two cases were 3 + on mucosal biopsies and 0 on resection. Of the four cases with difference in the HER2 score between mucosal biopsy and resection, one case showed HER2 score of 0 on mucosal and 1+ on resection making them negative. In the other three matched samples, there was a discrepancy of 0 and 3+ making the discordance significant.
Since five cases showed HER2 score 3+ on both biopsy and resection and three cases showed HER2 score 3+ in either mucosal biopsy or resection, a total of eight cases of HER2 positive were obtained. Among the eight HER2 positive (3 +) cases, five cases showed good concordance between resection and mucosal biopsies [Table/Fig-2]. Three cases showed discordance between mucosal biopsy and resection specimen [Table/Fig-3].
HER2 staining characteristics of positive concordant cases.
| Case | Age | Sex | Lauren classification | Grade (AJCC-2017) [9] | NACT | Biopsy score | Resection score | Heterogeneous staining on resection | Final HER2 |
|---|
| 1 | 55 | M | Intestinal | Moderate | NO | 3+ | 3+ | Present (80%+Ve) | Positive |
| 2 | 32 | F | Diffuse | Poor | NO | 3+ | 3+ | Present (10%+Ve) | Positive |
| 3 | 66 | M | Diffuse | Poor | NO | 3+ | 3+ | Present (10%+Ve) | Positive |
| 4 | 57 | F | Intestinal | Moderate | NO | 3+ | 3+ | Absent | Positive |
| 5 | 35 | F | Intestinal | Moderate | NO | 3+ | 3+ | Absent | Positive |
Characteristics of cases exhibiting discordance of HER2 staining in mucosal biopsies and resection specimen.
| Case | Age | Sex | Lauren classification | Grade (AJCC-2017) [9] | NACT | Regression Grade | Biopsy score | Resection score | Heterogeneous staining on resection | Final HER2 |
|---|
| 1 | 24 | M | Diffuse | Poor | Yes | 3 (Poor) | 0 | 3+ | Present (30%+ve) | Positive |
| 2 | 53 | M | Diffuse | Poor | Yes | 2 (Minimal) | 3+ | 0 | Absent | Positive |
| 3 | 63 | M | Intestinal | Moderate | Yes | 1 (Moderate) | 3+ | 0 | Absent | Positive |
All the three discordant cases had received NACT and the five concordant cases had not received NACT. All the three discordant cases showed heterogeneous staining pattern of HER2 on the resection specimen [Table/Fig-4]. Two cases showed a negative shift in which mucosal biopsy showed HER2 positivity score 3+ and the matched resection showed a HER2 score 0. One case showed a positive shift i.e. the biopsy showed a HER2 score 0 but resection showed HER2 score 3+ [Table/Fig-5]. There was no association between HER2 staining with Lauren’s type (p=0.572) or histologic grading (p=0.34).
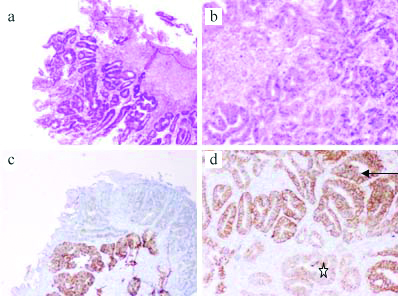
Case with concordant HER2 staining: a) Mucosal biopsy with adenocarcinoma at 10x magnification, H&E stain. b) Gastric surgical resection specimen with adenocarcinoma at 40x magnification; H&E stain. c and d) HER2 Immunohistochemistry (IHC) positive staining on both biopsy (C-10x magnification) and resection (D-40x magnification); the HER2 stain shows a heterogeneous staining pattern, HER2 negative* and HER2 positive areas.
(This image is part of the thesis submitted in partial fulfillment for Dr. Anand’s Post graduation, available in repository).
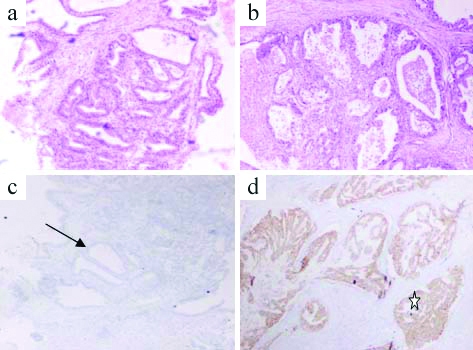
Case with discordant HER2 staining: a) Mucosal biopsy with adenocarcinoma at 10x magnification, H&E stain; b) Surgical resection specimen with adenocarcinoma at 40x magnification; H&E stain; c) HER2 Immunohistochemistry (IHC) score 0 on mucosal biopsy; d) HER2 Immunohistochemistry (IHC) score 3+ on surgical resection*.
Discussion
Gastric adenocarcinoma is the second most common cancer reported worldwide [10]. The HER2 oncogene is a transmembrane tyrosine-kinase receptor encoding cell proliferation and survival that has been shown to be involved in the pathogenesis of some gastric adenocarcinomas [3]. Trastuzumab is used as a targeted therapy against HER2 in cases of advanced gastric adenocarcinoma with significant positive survival in patients who showed human HER2 gene overexpression by IHC [5]. Upper gastrointestinal endoscopy and mucosal biopsies are crucial in the diagnosis and management of gastric adenocarcinomas, but there is little evidence that mucosal biopsies are sufficient to evaluate HER2 overexpression when compared with surgical resections that have much greater volume of tumour. Authors have studied HER2 expression in matched mucosal biopsies and surgical specimens of patients with gastric adenocarcinoma to compare their usefulness in the evaluation of HER2 overexpression.
Authors did not find any association between HER2 overexpression and age or gender of the patient although Matsusaka S et al., and Fan XS et al., showed a statistically significant correlation of increased expression with the male gender [13,14]. In the present study, no association was found between HER2 positivity and the type of gastric cancer (intestinal versus diffuse) or grade of tumour (moderate versus poorly differentiated) as reported by other authors [4,14-21].
Authors found HER2 overexpression by IHC in 9.72% mucosal biopsies and 8.3% of matched surgical resections of 72 patients. The concordance rate between biopsies and resection in present study was 95.83%, almost similar to that found by Watson S et al., (GERCOR) and Pirrelli M et al., who had a concordance rate of 94% and 91.8% respectively [15,22].
In the present study, out of the eight HER2 3+ positive cases, five cases showed concordance and three cases showed discordance between biopsies and resection. Three of our concordant cases and one of our discordant cases showed a heterogeneous staining pattern with 10-80% tumour cells showing positive staining with HER2 in the resection specimen. This was similar to the study conducted by Ahn S et al., in which heterogeneity was defined as 10-90% tumour cells staining positive for HER2 [12]. The reasons for HER2 heterogeneity is not known and could be due to the presence of neoplastic clones where HER2 is overexpressed in an otherwise HER2 negative tumour or due to the focal silencing of HER2 expression in tumour areas where there is otherwise homogenous amplification of HER2 [11].
Two of our discordant cases showed a negative shift of HER2 expression. This could possibly be due to cold ischemia of the resection specimen, over/under fixation of the tissue or the effect of NACT [11]. Other studies however have shown a higher HER2 positivity in biopsies compared to surgical specimens [5,15,19,20] possibly because of better fixation of small volumes of tissue and minimal cold ischemic time in small biopsies when compared with resection specimen. Some of these studies however did not use the same patient’s biopsy and resection specimens for comparison [19,20].
The low prevalence of HER2 overexpression in the present study (11.1%) could be because HER2 positivity has been reported to be more common in GEJ tumours when compared to gastric carcinoma [20], and present study was limited to gastric tumours alone.
Limitation(s)
The number of patients enrolled in this study was relatively small. Larger numbers are needed to substantiate the findings of the present study.
GEJ tumours were not included in this study.
No HER2 2+ positive cases were found in this study and hence Fluorescence In Situ Hybridization (FISH) was not performed on any cases.
Conclusion(s)
To our knowledge, this was the first study to compare HER2 expression in gastric adenocarcinoma in matched mucosal biopsies and the corresponding resections in India. There was concordance of HER2 expression in 69 cases and discordance in three. Differences between biopsy and resection HER2 expression could be explained by intratumoural heterogeneity, cold ischaemic time, over/under fixation and possibly by decreased HER2 expression after NACT. The HER2 analysis by IHC on mucosal biopsy may be sufficient in the majority of cases, but evaluation of resections may be useful in a small number of cases where the mucosal biopsy is negative, to optimise the selection of trastuzumab-eligible patients, particularly following NACT. Studies on larger numbers of cases are required to substantiate these findings.