Recurrent Benign Phyllodes Presenting as High Grade Malignancy: A Case Report
Aditya Mehta1, Roshni Manek2, D Suhas3
1 Senior Resident, Department of General Surgery, AVBRH and Datta Meghe Institute of Medical Sciences, (Deemed University), Wardha Sawangi (Meghe), Maharashtra, India.
2 Junior Resident, Department of General Surgery, AVBRH and Datta Meghe Institute of Medical Sciences, (Deemed University), Wardha Sawangi (Meghe), Maharashtra, India.
3 Junior Resident, Department of General Surgery, AVBRH and Datta Meghe Institute of Medical Sciences, (Deemed University), Wardha Sawangi (Meghe), Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Roshni Manek, Radhikabai PG Girls Hostel, Sawangi (Meghe), Wardha, Maharashtra, India.
E-mail: roshanismanekk@yahoo.com
Johannes Muller initially described Phyllodes Tumours (PT) to be “cystosarcoma phyllodes” in 1838. The connotation for the term “phyllodes” is leaf like, which on pathologic examination gives appearance of typical papillary projections. The PT are fibroepithelial in nature and account for an unbefitting 1% of overall primary neoplasms of the breast. They are known for its rapid growth and seen in women of 35-55 years of age, with a mean age of presentation as 45 years. Regardless of the histology recurrence is very common, in both malignant and benign PT. The stromal pattern of expression of CD10 marker strongly correlates with the grade of PT thus helps in the differentiating between malignant and benign variants of PT. Authors hereby, discuss a case of 48-year-old female patient with recurrent PT of breast who underwent surgical excision of benign phyllodes tumour twice and presented with a briskly growing recurrent lump in the left breast for the third time, diagnosed as malignant PT. Very finite data is available on the outcome of surgical management and the advantage of adjuvant Radiation Therapy (RT) in PT. The PT carry high risk of recurrence as well as become aggressive when malignant in nature. Thus, wide local excision of the tumour with negative margins should be followed by adjuvant chemotherapy and radiotherapy to decrease the recurrence rate.
Adjuvant radiotherapy, Breast, Recurrence, Surgery
Case Report
A 48-year-old female patient presented with a complaint of lump in upper inner quadrant of left breast three months back. The lump was initially of size approximately 3×2 cm at onset and rapidly progressed to the size of 8×7 cm in a period of three months. The lump was not associated with any aggravating or relieving factors. Patient did not give history of backache, breathlessness, pain over the swelling, loss of weight or appetite. Patient underwent excision of similar lump in left breast on same site of current presentation. The histopathological report of the excised lump, at primary presentation was suggestive of benign PT. One and half year after primary lump excision patient developed first reccurence of lump at same site over left breast at 11’O clock position, which was excised again at another hospital. The histopthology report of the tumour excised after first recurrence was suggestive of malignant PT. In this case, the first local recurrence was one and half years after initial excision and second recurrence was seen within three months of treatment of first recurrence.
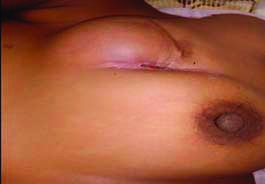
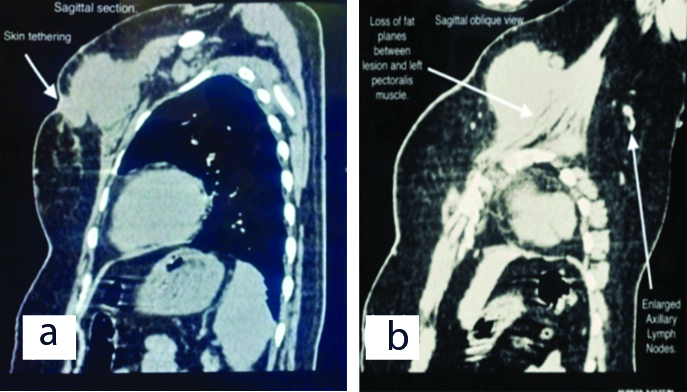
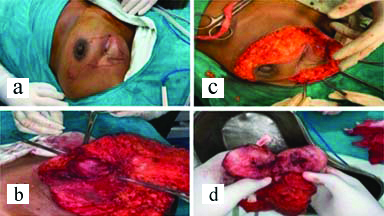
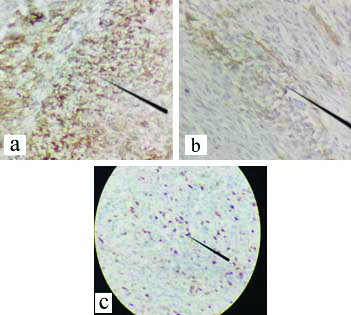
On examination left breast had a single swelling palpable in upper inner quadrant of left breast. The lump measured approximately 8×6 cm in size and fixed to the chest wall and pectoralis major muscle. Scar line was noted over the palpable lump with serous discharge and excoriation over the scar line [Table/Fig-1]. Routine blood investigations were done and were within normal limits. Ultrasonography of bilateral breast with axilla was suggestive of solid appearing mass in left breast with low level internal echoes suggestive of recurrent PT. High Resolution Computed Tomography (HRCT) thorax showed following features of large well defined lobulated isodense mass lesion in the upper and inner quadrant of the left breast opposite to first and second ribs [Table/Fig-2a,b]. Loss of fat planes between the lesion and left pectoralis muscle with associated retraction of the overlying skin and few enlarged lymphnodes in the bilateral axilla largest measuring 1.5×1.7 cm [Table/Fig-2b]. All the features were suggesting the possibility of recurrence of PT. Metastatic workup (ultrasonography of abdomen and pelvis, X-ray of chest and thoracolumbar spine) was done which was unremarkable. Fine Needle Aspiration Cytology (FNAC) was done from the lump in the left breast which was suggestive of malignant PT. The patient underwent left simple mastectomy with left axillary lymphnode sampling [Table/Fig-3a,b,c]. The challenge faced for performing the surgery was the nature of incision to be taken, as the patient already had previous scars with skin involvement and tumour being in proximity to the clavicular region provided limited margin for dissection of the tumour, thus making primary closure difficult. An irregular elliptical incision was taken involving the tumour and nipple areolar complex of the left breast as seen in [Table/Fig-3a-c]. The specimen obtained was then sent for histopathological examination and immunohistochemistry [Table/Fig-3d]. Primary closure was done with no evidence of infection and flap necrosis postoperatively. Histopathology report was suggestive of malignant phylloides tumour of left breast with no nodal metastasis. Also immunohistochemistry was suggestive of malignant PT, immunoreactive for CD-10, focally moderate positive for Smooth Muscle Actin (SMA) and immunonegative for Pan-Cytokeratin (Pan-CK), p63 and C-kit. The MIB-1 labelling index was 70% [Table/Fig-4a-c]. Postoperatively, the patient received four cycles of chemotherapy with adriamycin+iphosphamide+mesna followed by radiotherapy with 3D Conformal Radiation Therapy (3DCRT) (Truebeam STx Linear Accelerator). A dose of 50Gy/25 fractions was delivered in a period of 35 days with symptomatic treatment given during the course of radiotherapy. Follow-up was taken six months post surgery and the patient showed no signs of recurrence and had healthy scar.
Clinical picture of lump over the left breast at the time of presentation.
a) Sagittal section of High Resolution Computed Tomography (HRCT) showing skin tethering; b) Sagittal-oblique view of HRCT showing loss of fat planes and enlarged axillary lymphnode.
a) Incision taken for simple mastectomy in this patient; b) Attachment of tumour to the pectoralis muscle; c) Breast tissue and tumour separated from the overlying skin; d) Cut section of tumour and specimen sent for histopathological examination and immunohistochemistry.
a) Tumour cells showing diffuse and strong immunoreactivity for CD10 (IHC, 40x); b) Tumour cells showing patchy immunoreactivity for SMA (IHC, 40x); c) Ki 67/MIB labeling index was 70% (IHC, 10x).
Discussion
Johannes Muller initially described PT to be “cystosarcoma phyllodes” in 1838. The connotation for the term “phyllodes” is leaf-like, which on pathologic examination gives appearance of typical papillary projections [1]. PT are commonly seen in women of middle age, having mean size of 4-7 cm with expeditious growth [2]. They are fibroepithelial in nature and account for unbefitting 1% of overall primary neoplasms of the breast [3]. PT are known for its rapid growth and seen in women of 35-55 years of age, with a mean age of presentation as 45 years [2]. The transformation of PT from benign to a malignant tumour is not very common and is also unpredictable [4]. PTs is classified by The World Health Organisation (WHO) into three subtypes depending upon its histological characteristics of stromal cell mitotic activity, stromal cellularity, stromal overgrowth, stromal nuclear atypia, type of borders (infiltrating or pushing) and tumour necrosis as: benign, borderline, and malignant [5]. The local recurrence is seen within the first few years after surgery, especially when incomplete excision was performed initially [4]. PT are mostly benign, thus major concern postsurgery is recuurence: local vs. distant recurrence [6]. Around 15% of PT have a likelihood to recur locally, whereas fewer tumours develop metastasis to distant sites. Even though, histotype is considered the most important prognostic factor, there are reports which suggest that it has less importance than margin status while predicting local recurrence [7].
In the present case, the first local recurrence, was one and half years after initial excision and second recurrence was seen within three months of treatment of first recurrence. As per various studies, the backbone of curative treatment of PT is surgery that includes breast conservation surgery and mastectomy [5]. The treatment for locally recurring tumour is re-excising of the tumour which is superseded by radiotherapy [1]. According to recent guidelines PT of >3 cm should undergo resection with free margins of ≥1 cm; axillary staging is not required [2]. A study suggest role of chemotherapy in metastatic PT and radiotherapy for malignant, margin positive PT, to achieve local control [2]. A study carried out on 3120 patients in the American College of Surgeons’ published in the National Cancer Database and the data from this study was suggestive that radiotherapy can decrease the local recurrence rate and extend the time to local recurrence, with no significant influence on survival [5]. In the current case, left simple mastectomy with left axillary lymphnode sampling was done and four cycles of adjuvant chemotherapy with adriamycin and iphosphamide with mesna was given to the patient. This was followed by radiotherapy (50 Gy via 3DCRT) and the patient currently after six months of the surgery patient shows has no signs of local recurrence until six months postoperatively.
Histologically, it may be challenging to distinguish PT from fibroadenoma. However, distinction of malignant PT from spindle cell metaplastic carcinoma and primary breast sarcoma is difficult. In some instances, fibroadenomas having intracanalicular pattern of growth and stromal hypercellularity can mimic PTs [8].
Studies show that, as the lesions progress from benign PT to borderline PT to frankly malignant PT there is a marked increase in the expression of CD10 marker. All the cases having malignant PT showed intensity for CD10 to be strong and diffuse. All cases with borderline PT showed patchy and strong immunoreaction, while cases with benign PT showed weak and patchy immunoreactions. The stromal pattern of expression of CD10 marker strongly correlates with the grade of PT, thus helps in the differentiating between malignant and benign variants of PT and can assist the histopathological examination and grading the PTs accurately thus helping in careful planning treatment and follow-up. Sarcomatoid metaplastic carcinomas contain spindled component which stain positive for p63 or high molecular weight keratin and thus they can be differentiated from malignant PT [9]. In the present case, the tumour was immunoreactive for CD10, focally moderate positive for SMA and immunonegative for Pan CK, p63 and C-kit.
Study done by Chan YJ et al., states that Ki-67 antigen in the cell is related to proliferation which can be labelled along with monoclonal antibody MIB-1. MIB-1 immunostaining when applied on tissue sections helps in assessment of proliferative activity of tumours, especially those which include breast carcinoma. Ki-67 antigen (MIB-1 index) is defined as the percentage (%) of positivity in nuclear staining after we have counted one thousand neoplastic stromal cells. In benign lesions, the percentage of positive cells in the MIB-1 index is low and the percentage increases in malignant tumours [10]. In this case, the MIB labeling index was 70% which is suggestive of highly malignant tumour.
Conclusion(s)
The PT carry high risk of recurrence as well as become aggressive when malignant in nature. Thus, wide local excision of the tumour with negative margins, should be followed by adjuvant chemotherapy and radiotherapy to decrease the recurrence rate. However, more evidence is needed to prove the efficacy of adjuvant radiotherapy to prevent recurrence in a case of malignant PT.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 10, 2021
Manual Googling: May 13, 2021
iThenticate Software: Sep 06, 2021 (7%)
[1]. Kim S, Oh HY, Ryu Y, Benign phyllodes tumour of the breast recurring as a rapidly growing recurrent malignant phyllodes tumour: A case reportIran J Radiol 2019 16(2):01-06.10.5812/iranjradiol.13062 [Google Scholar] [CrossRef]
[2]. Garlet BB, Zogbi L, Lima JP, Favalli PPS, Krahe FD, Recurrent borderline phyllodestumour of the breast submitted to mastectomy and immediate reconstruction: Case reportInt J Surg Case Rep 2019 60:25-29.10.1016/j.ijscr.2019.05.03231195364 [Google Scholar] [CrossRef] [PubMed]
[3]. Park HJ, Ryu HS, Kim K, Shin KH, Han W, Noh DY, Risk factors for recurrence of malignant phyllodes tumours of the breastIn Vivo 2018 33(1):263-69.10.21873/invivo.1147030587634 [Google Scholar] [CrossRef] [PubMed]
[4]. Pornchai S, Chirappapha P, Pipatsakulroj W, Lertsithichai P, Vassanasiri W, Sitathanee C, Malignant transformation of phyllodes tumour: A case report and review of literatureClinical Case Reports 2018 6(4):678-85.10.1002/ccr3.142829636939 [Google Scholar] [CrossRef] [PubMed]
[5]. Chao X, Chen K, Zeng J, Bi Z, Guo M, Chen Y, Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumours: A systematic review and meta-analysisBMC Cancer 2019 19(1):372:1-7.10.1186/s12885-019-5585-531014268 [Google Scholar] [CrossRef] [PubMed]
[6]. Roberts N, Runk DM, Aggressive malignant phyllodes tumourInternational Journal of Surgery Case Reports 2015 8:161-65.10.1016/j.ijscr.2014.12.04125697402 [Google Scholar] [CrossRef] [PubMed]
[7]. Wei J, Tan YT, Cai YC, Yuan ZY, Yang D, Wang SS, Predictive factors for the local recurrence and distant metastasis of phyllodes tumours of the breast: A retrospective analysis of 192 cases at a single centerChinese Journal of Cancer 2014 33(10):492-500.10.5732/cjc.014.10048 [Google Scholar] [CrossRef]
[8]. Tan PH, Tse GMK, Yip GWC, Bay BH, Phyllodes Tumours of the Breast: The Role of Immunohistochemistry in DiagnosisMethods of Cancer Diagnosis, Therapy and Prognosis:251-262.10.1007/978-1-4020-8369-3_19 [Google Scholar] [CrossRef]
[9]. Kulkarni MM, Khandeparkar SG, Joshi AR, Kothikar V, Nasare A, Patil S, Role of CD10 Immunoexpression in Grading Phyllodes Tumour of the BreastJ Clin Diagn Res 2017 11(1):14-16.10.7860/JCDR/2017/25613.923128273972 [Google Scholar] [CrossRef] [PubMed]
[10]. Chan YJ, Chen BF, Chang CL, Yang TL, Fan CC, Expression of p53 protien and Ki-67 antigen in phyllodes tumour of the breastJ Chin Med Assoc 2004 67(1):03-08. [Google Scholar]