The BCC which is now the most common malignant neoplasm in human beings was first described by Jacob Arthur in 1827 [1]. The BCCs are currently hypothesised to originate from pluripotent cells in the basal layer of the epidermis or the follicle [2]. These tumours are seen on both, sun-protected and sun-exposed areas, but are postulated to have a different biology and morphology in these locations [3]. The BCC typically occurs in the fourth decade of life and beyond although exceptions to this are rarely seen. In younger patients, BCC is usually seen in the setting of specific genetic defects or in an immunocompromised state due to a defective immune surveillance of oncogenic viruses [4-6]. Exposure to arsenic, coal tar and radiation are additionally described risk factors [3].
The tumour is slowly progressive with low metastatic potential but, considerable local destruction and disfigurement may be seen due to delayed or inadequate treatment [7].
The clinical presentation of BCC is varied but it most commonly presents as a slow growing, well defined telengiectatic papule or nodule, above the line connecting the angle of the mouth and ear lobe. The most common clinical type is the nodular-ulcerative type, seen in sun-exposed areas of the head and neck, followed by the superficial subtype, which is most commonly seen in the trunk region. The diagnosis is usually clinical and confirmation is by biopsy and histopathological examination, which aids in determination of the histological subtype, pattern of growth and level of tumour invasion [8].
The microscopic subtypes include solid or nodular, micronodular, infiltrating, superficial, fibroepithelial, basosquamous or metatypical, keratotic, BCC with adnexal differentiation, and other rare variants [9,10].
The different histomorphological subgroups have their specific clinical symptoms pertaining to site and behaviour [11]. The histomorphological pattern is also one of the proven predictors of clinical behaviour of BCC [12].
The present study was aimed at augmenting the existing literature on BCC by analysing the clinical data of patients with BCC with respect to age, sex, site and type of lesion and the histological spectrum of BCC prevalent in the population of Mysore in Southern Karnataka including the uncommon variants.
Materials and Methods
An 11 year retrospective descriptive, observational study was carried out in the Pathology Department of a tertiary care teaching hospital in Southern Karnataka, India. All BCCs diagnosed in the Department of Pathology from January 2010 to January 2021 formed the basis of this study. Data thus collected was analysed between February and May 2021.
Inclusion criteria: All histologically proven biopsies of BCC were included in the study.
Exclusion criteria: The cases where biopsy was fragmentary and tumour tissue was minimal were excluded from the study.
Detailed clinical data of the patients including age and gender of the patient was recorded from the biopsy request forms and the clinical workstation.
Findings from clinical examination of the lesions with reference to site, number and colour of the lesions were also noted. Slides stained with haematoxylin and eosin was reviewed by the study pathologists and the histopathological diagnosis was confirmed of these slides. Some of the tumours were reclassified according to the new World Health Organisation (WHO) Classification of Skin Tumours, published in 2018 [9]. Since, it was a retrospective study from archival data, ethical clearance was not needed.
Statistical Analysis
Data was entered in Microsoft excel spreadsheet. The basic demographic data was summarised using descriptive statistics. Proportions were described as percentages.
Results
A total of 65 BCC lesions in 64 patients were diagnosed during the study period with a male to female ratio of 0.9:1. The age of the patients ranged from 21 to 82 years with a peak incidence in the sixth decade and the mean age was 61 years [Table/Fig-1].
Age and gender distribution of patients with BCC (N=64).
| Age range (years) | No of cases | Percentage of cases (%) | Males | Females |
|---|
| 0-9 | 0 | 0 | 0 | 0 |
| 10-19 | 0 | 0 | 0 | 0 |
| 20-29 | 2 | 3.1 | 1 | 1 |
| 30-39 | 3 | 4.7 | 1 | 2 |
| 40-49 | 6 | 9.4 | 2 | 4 |
| 50-59 | 17 | 26.6 | 9 | 8 |
| 60-69 | 12 | 18.7 | 6 | 6 |
| 70-79 | 15 | 23.4 | 9 | 6 |
| 80-89 | 9 | 14.1 | 2 | 7 |
All patients had complete excision of the tumour with no involvement of surgical margins in any case.
Most patients (63/64) had a single lesion. One patient was a case of oculocutaneous albinism and had two lesions. One of these was diagnosed as basosquamous carcinoma and the other as BCC with mixed histology. None of the patients had documented history of use of photoprotective sunscreens or protective clothing. There was no history of treatment with Psoralen (P) and long-wave Ultraviolet (UVA) or Narrow-band ultraviolet B (NB-UVB) in any of the study cases. Head and neck lesions were the most common, comprising 49 tumours (75.38%) and the distribution of lesions was as shown in [Table/Fig-2]. Uncommon sites noted included vulva and axilla.
Anatomical distribution of Basal Cell Carcinomas (BCC) (N=65).
| Site of lesion | N (%) |
|---|
| Forehead | 16 (24.62%) |
| Cheek | 10 (15.38%) |
| Nose | 8 (12.31%) |
| Pre-auricular area | 5 (7.69%) |
| Orbital area | 5 (7.69%) |
| Scalp | 4 (6.15%) |
| Upper lip | 1 (1.54%) |
| Anterior chest wall | 5 (7.69%) |
| Lower leg | 4 (6.15%) |
| Shoulder | 3 (4.62%) |
| Vulva | 2 (3.08%) |
| Axilla | 2 (3.08%) |
A total of 30/65 or 46.15% of BCCs presented as an irregular plaque followed by presentation as an ulcerative lesion in 22 cases (33.84%) [Table/Fig-3]. Other presentations included an erythematous plaque, hyperpigmented papule and a solitary nodule [Table/Fig-3].
Types of clinical presentation of lesions (N=65).
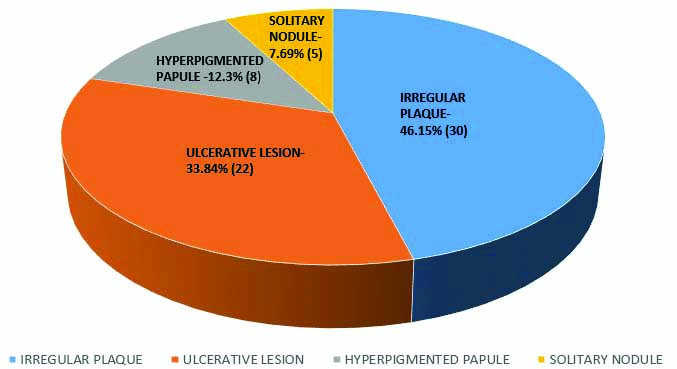
Pigmentation was noted in 18 (28%) of cases clinically. The most common clinical differential diagnoses in the pigmented lesions were melanoma in 10 out of 18 cases (55.55%).
[Table/Fig-4] shows the distribution of the various histopathological subtypes. Majority of the BCCs were of the nodular/solid type (36/65 or 55.38%) [Table/Fig-5] on histopathology, followed by the superficial/multifocal BCC (16/65 or 24.62%). The other variants noted were adenoid variant of BCC, metatypical/basosquamous BCC [Table/Fig-6] and one case each of BCC with mixed histology [Table/Fig-7] and BCC with sebaceous differentiation [Table/Fig-8].
Histological types of BCC (sum=65).
| Histological type of BCC | No. of cases (n=65) | Percentage |
|---|
| Nodular/Solid BCC | 36 | 55.38% |
| Multifocal/Superficial BCC | 16 | 24.62% |
| Adenoid variant of BCC | 7 | 10.77% |
| Metatypical/Basosquamous BCC | 4 | 6.15% |
| BCC with sebaceous differentiation | 1 | 1.54% |
| BCC with mixed histology | 1 | 1.54% |
Microscopy of nodular/solid BCC showing nodules of basaloid cells with peripheral palisading of cells. (H&E, 100x and 400x).
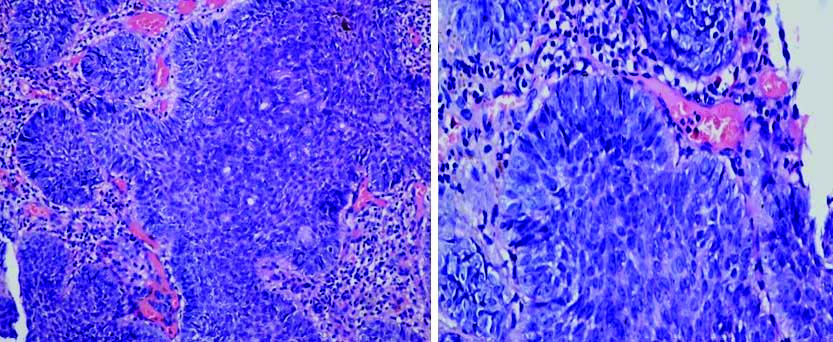
Microscopy of BCC with of basosquamous BCC showing large polygonal squamoid cells another portion of the tumour displaying trabecular growth of basaloid cells (H&E, 100x).
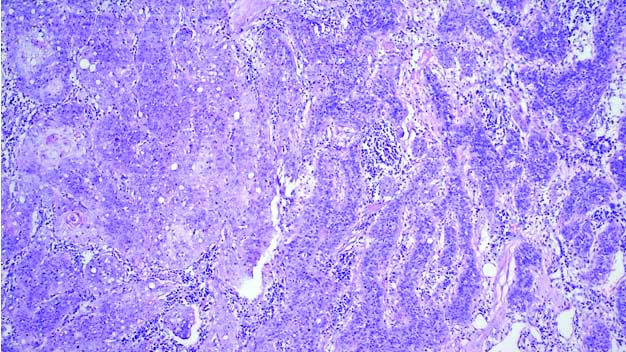
Microscopy of BCC with mixed histology showing nodular and cribriform architecture (adenoid and nodular variants of BCC) (H&E, 100x).
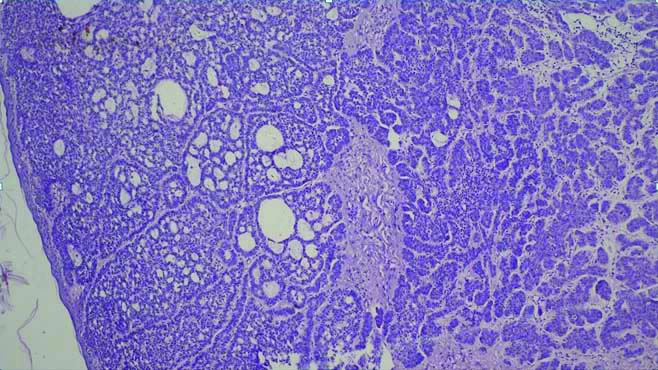
Microscopy of BCC with sebaceous differentiation (H&E, 100x).
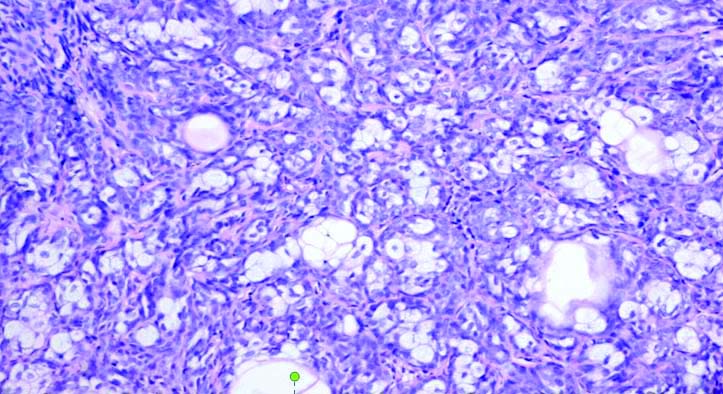
The tumour with mixed histology showed both classic nodular architecture and adenoid differentiation. Of the five recurrent BCCs, three were of nodular subtype and the other two tumours were multifocal/superficial (1) and basosquamous subtypes (1).
Though there were 18 clinically apparent pigmented lesions, 19 tumours showed microscopic evidence of melanisation.
Among the BCCs with microscopic evidence of pigmentation, the histological types were as follows- Nodular/solid (10 or 52.63% of cases), multifocal/superficial (4 or 21.05% of cases), BCC with adenoid pattern (3 or 15.79% of cases) and BCC with sebaceous differentiation (1 or 5.3% of pigmented BCCs). One case of multifocal BCC (5.3%) which clinically presented as a non pigmented lesion showed microscopic evidence of melanisation.
One BCC in our study which presented as a pigmented lesion had a co-existing intradermal naevus. Lymphovascular and perineural invasion were seen in one tumour which also showed metastasis to the cervical lymph nodes. Necrosis was observed in two tumours and a foreign body giant cell reaction was seen in one tumour. Two tumours, both of basosquamous type [Table/Fig-7] showed tumour invading the deep dermis with one of them showing infiltration into subcutaneous tissue as well. Another rare variant encountered was BCC with sebaceous differentiation [Table/Fig-8].
Discussion
The BCC is the most common malignant cutaneous neoplasm worldwide and although incidence rates of BCC vary according to ethnicity and geographic location, occurrence seems to be increasing worldwide in the past few decades [13]. This noteworthy increase of the incidence in BCC in recent decades has inspired the phrase “silent epidemic of the twentieth century” [14]. This has been attributed to a combination of multiple factors, which include increased exposure to UV light, depletion of the ozone layer, and increased awareness and surveillance [15].
When the age distribution was analysed, in present study study, almost 83% of cases were of patients older than 50 years of age with most cases seen in the sixth and eighth decades (26.6% and 23.4% of cases respectively). Previous studies have also shown that majority of BCC are seen in individuals aged 60 years or older [5,16]. A similar peak has been noted in the seventh and eighth decades by some authors [14,17] while Aandani A and Ganatra MA have found BCC to be more prevalent in fifth decade in their study [18]. The most accepted hypothesis for higher incidence of BCC in elderly patients is a less robust immune system with diminished capacity for repair of DNA damage which increases the risk of developing skin cancers including BCC [19]. Age too, does not seem to be a discriminant factor for any particular histologic pattern of BCC [20].
Studies by Bastiaens MT et al., and Raasch BA et al., have reported a male preponderance of BCC with studies with the percentage of male patients being 54%, and 58.6%, respectively [21,22].
Previously, BCCs were more commonly observed in males, purportedly because of more occupational and recreational exposure to UVA but recent trends show a slight increase in female patients [14]. The present study showed a very slight female preponderance which is in concordance with findings from another Indian series [23]. The exposure of Indian women, particularly in rural areas to intermittent, high intensity UVA while working in open kitchens and fields might explain the female preponderance in the present study study, as intermittent rather than continuous cumulative exposure to UVR has been implicated in the pathogenesis of BCC [24]. It has also been observed that BCCs in women are of smaller size at diagnosis and this carries a clinical relevance as females tend to seek medical care earlier than males for suspicious, asymptomatic, and cosmetically disfiguring lesions [25]. This data however could not be related in the present study.
In the present study, five tumours were noted in patients younger than 40 years, of which two were male and three were female and the low incidence of this tumour in this age group is in concordance with other studies [5]. The youngest age of presentation in case of females was 21 years, while the corresponding age in males was 24 years. The clinical suspicion in three of these cases was seborrhoeic keratosis (two cases) and porokeratosis (one case) likely due to the rarity of BCC in this age group. The BCC in young patients is often diagnosed only on final histopathological examination of an excisional specimen causing a delay in treatment due to a low index of suspicion owing to the low incidence of this tumour in young patients [26].
Authors have also found that BCC in young patients has a female preponderance especially in those who have history of past or current smoking [27]. There is no definite explanation for the rising incidence of BCC in young people but it is likely that increasing exposure to UVA plays a causal role [28]. The BCC in the paediatric population is extremely rare and is usually seen in children with predisposing genetic conditions [29]. In the present study, we did not encounter any patients in the paediatric age group.
In concordance with other studies, the most common site of BCC was the head and neck followed by the trunk and the lower limb [30,31]. This anatomical distribution corresponds to the intensity of exposure to sunlight where the highest density of lesions were seen on body sites frequently exposed to sunlight in supporting the implication of sun-exposure as the primary aetiologic factor for these tumours [31].
The lesions on sun-protected sites in our study included the thigh and vulva. Authors have shown that vulvar BCC is not an exceptionally uncommon entity and comprises about 2-4% of all cases of vulvar malignant tumours [32,33]. The aetiopathogenesis of BCC in sun-protected areas remains mired in controversy and factors other than UVA like radiotherapy to the pelvic region, chronic inflammation and previous burn or scar have been implicated in the development of BCC in these sites [34-36].
The most common clinical presentation of BCC was as an irregular plaque in concordance with other studies [5]. A significant percentage of BCCs were clinically pigmented (28%) and a pitfall also reported in other studies [37,38] was the mistaken diagnosis of most of these lesions as tumours of melanocytic origin (61% of pigmented tumours). Although BCC can be suspected from features like pearly appearance with telangiectasia in a papular or nodular lesion, these features may be subtle and confirmation of diagnosis requires histopathologic examination of biopsy [39]. The proportion of BCCs presenting as pigmented lesions was in concordance with another study by Poppe L et al., but much lower than the proportion reported in an Indian study [10,40].
The common histological features of nodular or solid variant of BCC are solid, irregular, lobulated variably sized nests composed of basaloid tumour cells connected to epidermis displaying peripheral palisading of nuclei and clefting artefact between the epithelium with variable stromal infiltrates of inflammatory cells [14]. The most common morphological subtype in both males and females in the present study was the nodular/solid subtype (55.38%) similar to other studies [8,14,41,42]. Focal eccrine or sebaceous elements have been described in nodular BCCs and roughly one-third of cases have additionally shown a coexistent superficial component [1] but this was not observed in the present study.
Among the clinically pigmented BCCs, in the present study, the most common morphological subtype of BCC which presented as a pigmented lesion was the nodular/solid type (52.63% of cases) followed by multifocal/superficial type (4 or 21.05%). Some authors have found that the histologic type of BCC most frequently associated with pigment was the nodular/micronodular type [43,44], while others have found that nodular BCCs constituted the most frequent form of pigmented BCC [1]. While any histological variant of BCC can have pigment, morphoeic and infiltrative subtypes are found to be uncommonly pigmented [45].
Authors have noted that BCC with sebaceous differentiation, a rare variant of BCC, can clinically present as a hyperpigmented nodule [46,47] and this was seen in our study as well the patient with BCC with sebaceous differentiation presented as a solitary hyperpigmented nodule.
Authors have described clinically non pigmented BCC which had microscopic evidence of melanisation but common subtypes displaying this feature have not been mentioned [48]. In the present study, the authors found only one case of multifocal BCC which clinically presented as a non pigmented lesion with microscopic evidence of melanisation. Interestingly, we also found many pigmented lesions with only focal microscopic evidence of melanisation. Among these, one tumour showed prominent sebaceous differentiation and one showed sebaceous hyperplasia with an intradermal nevus.
Authors have found that there is no significant difference in age or gender between pigmented and non pigmented BCCs. They also found that in the superficial and micronodular subtypes, pigment was seen more as a component of the cells rather than in the dermis with the highest amount of pigment seen in the superficial/multicentric type [40]. A study also found that BCC with depths greater than 3.3 mm tended to have no microscopic pigmentation [49] but this could not be assessed in the present study.
The next most common histological type is the multifocal or superficial type (24.62%) similar to the proportion in other studies [50]. These tumours are frequently described in younger patients by authors [51] but the authors noticed only one young patient with this subtype in the present study. A significant number of cases of superficial BCC in our study presented as an erythematous plaque (46%) in concordance with a study where an erythematous patch or plaque was the most common clinical presentation of superficial BCC. This study also found that size of the tumour was significantly larger than other subtypes of BCC, but this data was not available in the present study. While most cases of multifocal/superficial BCC in the present study were found on the head and neck (63%) followed by trunk (19%) and lower limb (13%), authors have found the trunk was the most common area of involvement [52]. However, in the present study too, all the tumours (100%) which were seen on the trunk (3/65), were of multifocal/superficial type. Generally, prevalence of a nodular pattern for sun-exposed areas and of a superficial pattern for sun-unexposed areas has been observed [20].
On microscopy, these tumours consist of superficial small nests of basaloid cells connected to the epidermis, extending into the dermis surrounded by a loose myxoid stroma [9,10]. This represents horizontal spreading but these tumours may be aggressive too and one case of multifocal/superficial BCC in the present study was a recurrent tumour [53].
The next common variant is the metatypical or basosquamous variant which comprised 4/65 or 6.15% of the cases in the present study. The incidence of this variant is vague and studies have found that it represents approximately 6% to 12% of all BCCs [10,14] similar to the low proportion of this subtype in the present study. Metatypical carcinomas represent BCC with squamous differentiation and are considered an intermediate type between BCC and squamous cell carcinomas [54]. This variant has been observed to behave aggressively and is commonly associated with local or extensive metastases. Among the four cases of basosquamous variant of BCC seen in the present study, only one of them had necrosis, lymphovascular and perineural invasion and evidence of metastasis to the lymph nodes [14,53]. It has also been seen that micronodular, infiltrating, sclerosing, and basosquamous differentiation in BCC is associated with more aggressive behaviour with higher rates of invasion of the dermis [1]. In the present study too, both tumours with infiltration into the deep dermis showed basosquamous differentiation.
Majority of BCCs are relatively non aggressive and metastasis has been reported only in 0.0028-0.5% of all cases [55]. In the present study too, only the aforementioned BCC with basosquamous histology showed metastasis. Also, worthy of note was the clinical presentation of all the metatypical BCCs was as an ulcerated lesion and this may be due to the feature that aggressive growth tumours tend to show more frequent ulceration [1].
Only one case of adenoid variant of BCC was seen in the study and though there is little literature on the exact incidence of adenoid BCC, Bastiaens MT et al., has reported the incidence of 1.3% [21]. An important differential diagnosis of adenoid variant of BCC is adenoid cystic carcinoma and this is often a histopathological challenge, since in adenoid cystic carcinoma too, the tumour cells may be basaloid with hyperchromatic nuclei arranged in a cribriform pattern undergoing dedifferentiation within a collagenous stroma. However, the lack of peripheral palisading of the tumour cells in an adenoid cystic lesion without connection to the overlying epidermis and the absence of artefactual clefts between the tumour and the stroma help in differentiating between an adenoid cystic carcinoma and adenoid variant of BCC [56].
Multiple recurrences in BCC are associated with considerable morbidity. In the present study, five cases included were recurrent BCCs. Recurrence in BCCs are seen to be more common in lesions in the nasal area, which may be due to difficulty in securing adequate margins in this site. Accurate histological evaluation of relapsing BCCs is often formidable for the pathologist because of the altered morphology of the tumour by scar tissue which alters the original architecture of the tumour as well as the pattern of tissue infiltration [1].
Immunohistochemistry may be required to differentiate BCC from close histopathological mimics like trichoblastoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. Trichoblastoma stains positive for CD10 and CD34 whereas the BCC does not [57]. In the differentiation from trichoepithelioma, BCC expresses BerEP4, CK6 and Androgen Receptor (AR) all of which are negative in trichoepitheliomas. Finally, CD10 is positive in BCC tumour cells and positive in the stroma in tricoepithelioma [56].
A combination of CK15 and CK7 has been found to be useful in differentiating microcystic adnexal carcinoma from infiltrative BCC where the immunohistochemical profile of microcystic adnexal carcinoma was CK15+/CK7- or CK15+/CK7+ while infiltrative BCC would be CK15-/CK7+ [58].
Limitation(s)
Limitations of this study include lack of follow-up and absence of paediatric cases of BCC.
Conclusion(s)
This study reveals the recent trends in clinical and morphological aspects of BCC in the population of southern Karnataka, India. The clinical and histological data on BCCs in the present study is in agreement with that in literature in many aspects. The clinical and histopathological diagnosis related well in 54 (83%) of biopsies. Histopathological evaluation of BCC is of paramount importance not only to establish diagnosis but also to predict behaviour and risk of recurrence. The results of the present study exhibit specific patterns in the age, anatomical distribution and clinical presentation of the different subtypes of BCC.