Chronic wounds cause significant morbidity and mortality. A major type of it is diabetic ulcer, which has a 25% lifetime risk of developing chronic wounds and is also associated with 43-55% 5-year mortality rate. Furthermore, it continues to be the leading cause of (80%) non traumatic lower limb amputation. The global prevalence of DFU is 6.3%. India has the largest diabetic population and is expected to increase by 2025 to 57 million [1]. Studies revealed recurrent foot infections are common among 52% of Indian diabetic patients [2]. On average, the cost of treating one DFU over two years is estimated to be $28000 [3]. Risk factors implicated in the development of diabetic ulcers are neuropathy, trauma, deformity, peripheral vascular disease, high plantar pressures, poor glycaemic control, smoking and ishaemia of small and large blood vessels.
Wound infection is associated with 90% of lower extremity amputations, especially in diabetes [4]. However, the infection may not be evident in the absence of clinical signs. In such instances, wound bioburden is the best indicator of infection. Studies have suggested that wounds with a high microbial load of greater than 105 Colony Forming Unit (CFU) per gram of tissue are considered critical for diagnosing infection and are associated with an increased incidence of wound sepsis [4-6].
In Biofilms, microbes exhibit synergistic relationships and alter virulence and pathogenicity. The fungi (mycobiome) act as a cofactor in the inflammatory process, form mixed fungal-bacterial biofilms, conferring reduced antibiotic sensitivity and poor wound healing. Hence, reduced antimicrobial susceptibility and inadequate eradicating infection in biofilms contribute to chronic, recalcitrant, recurrent non healing wounds. These factors led to the proposal of Biofilm-Based Wound Care (BBWC) [14]. Chronic wounds can be treated with proper wound debridement, appropriate selection of antibiotics based on the antibiogram of isolates, and local anti-biofilm agent application.
Hence, this study was aimed to determine the bacteriome and mycobiome of diabetic ulcers. Also, to determine the biofilm formation and the associated antimicrobial resistance profile of the pathogens and molecular characterisation of biofilm-forming resistant isolates by PCR.
Materials and Methods
This cross-sectional study was conducted on 150 (143 samples rounded to 150) diabetic ulcer patients from January-December 2019 in the Microbiology department at a Tertiary care hospital. Assuming the common isolate as Pseudomonas (16%) taken from previous literature [15] with precision 6%, power of 80% and confidence interval 95%, 150 samples were collected. Ethical clearance was obtained (IEC Approval No.13092018). Before the study, informed consent was obtained from the study population. Clinical data and complete haemogram findings were recorded.
Inclusion criteria: Diabetic ulcers in both type 1 and type 2 with age ≥ 20 years of both sex.
Exclusion criteria: Acute wound infections, postoperative wound infections, malignancy-associated ulcers and other chronic ulcers (pressure, vasculitic).
The ulcer site was cleansed and decontaminated with 10% povidone iodine and normal saline and samples such as tissue bits, pus, exudates were collected by rubbing the deepest accessible area ulcer covering an area of 1cm with two sterile swabs after adapting aseptic measures. One among the two swabs was used for culture and the other for gram staining. Tissue bits were collected aseptically in a sterile closed container containing normal saline without preservative (to keep the tissue moist) was transported within an hour, and homogenised in a tissue grinder or minced before mycological evaluation. The specimens were inoculated into Mac Conkey agar, Blood agar for bacteriological analysis and incubated at 36±1°C for 24-48 hours. In addition, the prepared specimens were inoculated into two tubes of Sabouraud Dextrose Agar and incubated at 25°C and 37°C, respectively. Tissue bits were collected in Robertson cooked meat broth and incubated at 36±1°C for 48 hours for anaerobic culture and processed using the Gas pack in a McIntosh fields jar. Bacterial and fungal pathogens were identified by direct microscopy, colony characterisation and biochemical parameters.
Biofilm detection by tissue culture plate method [17]: Colonies from fresh agar plates were inoculated onto trypticase soy broth with 1% glucose (10ml) and incubated at 37°C. A 24 hours later, the cultures were diluted 1:100 with a fresh medium, then 200 μL of diluted culture media was inoculated into 96 well flat bottomed polystyrene microtiter plate. Controls were set up as follows: Blank well, crystal violet, sterile trypticase soy broth, fixative respectively in the wells. Positive control: American Type Culture Collection (ATCC) Pseudomonasaeruginosa ATCC 27853 and Negative control: ATCC Staphylococcus aureus ATCC 25923.
Bacterial cells were grown in the wells after 24 hours of incubation. Wells then decanted, washed with Phosphate Buffer Saline (PBS) (0.2 mL) of pH 7.2, removing free-floating bacteria. Biofilm was fixed with 2% sodium acetate and stained with crystal violet (0.1%), then washed with deionised water removing excess stain and was air dried. Optical density measured at 570 nm with Enzyme Linked Immuno Sorbent Assay (ELISA).
Interpretation:
Based on the criteria of Stepanovic S et al., [18]
Optical Density (OD) value was calculated using the formula:
ODC (Optical density cut-off value)=Average OD of Negative control+3×(standard deviation of Negative control) [7].
Strong- >4 times ODC
Moderate- 2 times the ODC- 4 Times ODC
Weak- ≤2×ODC
Antimicrobial susceptibility testing: It performed by the Kirby-Bauer disc diffusion method. The test organisms grown on culture media were inoculated into peptone water and incubated at 37°C for 2-4 hours. The turbidity was matched with 0.5 McFarland. Lawn culture was made over Mueller-Hinton agar (MHA) plates and the antibiotic discs were placed according to the growth of gram positive or gram negative organisms. The plates were incubated for 18-24 hours at 37°C. The zone of diameter was recorded and interpreted as sensitive, intermediate, or resistant according to CLSI standards 2019 [19].
Colistin susceptibility testing: By broth microdilution for carbapenem-resistant isolates was performed as per Indian Council of Medical Research (ICMR) guidelines. Isolates with Minimum Inhibitory Concentration (MIC) ≤2 μg/mL were considered Susceptible and >2 μg/mL as Resistant to colistin [20].
Molecular characterisation: Biofilm-forming resistant isolates were done by PCR [21-23]. PCR was performed using PureFast® Bacterial DNA minispin purification kit purchased from HELINI Biomolecules, at Chennai, Tamil Nadu, India.
Bacterial DNA purification: Overnight culture (1 mL) centrifuged at 6000 rpm for 5 minutes and the supernatant was discarded. PBS (0.2 mL) was suspended with a pellet. Lysosome digestion buffer (180 μL) and 20 μL lysosome (10 mg/mL) were added and incubated at 37°C for 15 minutes.
A 400 μL of binding buffer was added with 5 μL of Internal control Template, 20 μL of proteinase K and Ethanol (300 μL) and mixed well. Above mixture was incubated for 15 minutes at 56°C. The entire sample was transferred into PureFast® spin column and centrifuged for 1 min. The flow-through was discarded and the collection tube was placed back and washed with wash Buffer 1, wash Buffer 2, centrifuging for 30-60 seconds each time, and the flow-through was discarded. The column was placed back and centrifuged for one minute. The spin column was transferred into a fresh 1.5 mL micro-centrifuge tube. A 100 μL of Elution Buffer added to center of spin column membrane, incubated at room temperature for 1 min and then centrifuged for 2 minutes. The column was discarded, and purified DNA was stored at -20°C.
PCR master mix: 2U of Taq DNA polymerase, 10X Taq reaction buffer, 1 μL of 10 mM dNTPs mix, 2 mM MgCl2, Red Dye PCR additives.
PCR primers mix [22,23]:
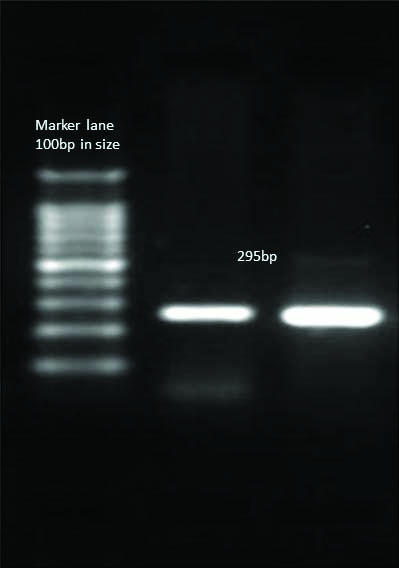
HELINI Ready to use blaCTX-M gene primer mix- 5 μL/reaction PCR Product: 295 bp
HELINI Ready to use blaNDM-1 gene primer mix- 5 μL/reaction PCR Product: 214 bp
HELINI Ready to use blaoxa-23 gene primer mix- 5 μL/reaction PCR Product: 360 bp
HELINI Ready to use mecA gene primer mix- 5 μL/reaction PCR Product: 220 bp
HELINI Ready to use blaTEM gene primer mix- 5 μL/reaction PCR Product: 260 bp
PCR Procedure:
| Components | Quantity |
| • HELINI RedDye PCR Master mix | 10 μL |
| • HELINI Ready to use- Primer Mix | 5 μL |
| • Purified Bacterial DNA | 10 μL |
| Total volume | 25 μL |
The components were added. After brief spinning, the programme was run as follows:

Agarose Gel Electrophoresis: To 2% Agarose (2 g Agarose in 100 mL of 1XTAE), 5 μL of Ethidium Bromide was added at 60°C. The solution was poured into a gel plate and placed undisturbed until it solidifies. Then, the gel plate was placed in a tank filled with 1X TAE Buffer.
PCR samples were loaded after mixing with gel loading dye along with a 10 μL HELINI 100 base pair (bp) ladder. {100 bp, 200 bp, 300 bp, 400 bp, 500 bp, 600 bp, 700 bp, 800 bp, 900 bp, 1000 bp and 1500 bp}. At 50 V, electrophoresis was rerun until the dye moved upto 3/4th distance of gel plate and viewed under Ultraviolet (UV) Transilluminator and observed for the band pattern.
Statistical Analysis
Quantitative variables, demographic variables, were expressed as a number, frequency, or arithmetic mean±SD, while qualitative variables were expressed as proportions or percentages.
Results
Out of 150 patients with non healing diabetic ulcers, 52 (34.7%) of cases belonged to the age group of 51-60 years followed by 48 (32%) in 41-50 years, 27 (18%) in 61-70 years, 14 (9.33%) in 71-80 years, 8 (5.3%) in 20-40 years and 1 (0.7%) in >80 years. Among 150 patients 80 (53.3%) were males and 70 (46.7%) were females. Patients with type 2 diabetes related ulcer were 136 (91%) than type 1 diabetes related ulcer, 14 (9%). 135 (90%) patients were associated with anaemia, followed by neuropathy 85 (56.7%), nephropathy 54 (36%), hypertension 40 (26.7%), as major complications related to diabetes [Table/Fig-1]. Overall, 26 (17.3%) of patients underwent limb amputation. Out of which, 18 (69.2%) were associated with anaemia risk factors. The most common sites of ulcers were the plantar surface of foot 68 (45.3%), toes 48 (32%), and dorsal surface of foot 34 (22.7%). A total of 142 patients showed microbial growth, either polymicrobial or monomicrobial. Monomicrobial aetiology was 73 (51.4%) and polymicrobial 69 (48.6%). Out of 216 isolates, gram negative aerobes 112 (52%) are predominant than gram positive aerobes 54 (25%). The rest of the growth showed 38 (18%) Fungal and 12 (5%) anaerobic growth. The predominantly isolated pathogens from 216 isolates were Pseudomonasaeruginosa 37 (17.1%) and Staphylococcus aureus 33 (15.2%) among aerobic bacteria, Peptostreptococcus 10 (4.6%) among the anaerobes and Candida albicans 20 (9.2%) were most predominantly isolated among fungus. Other infections among aerobes include Escherichia coli 17 (7.9%), Klebsiella species 22 (10.2%), Acinetobacter baumannii 15 (6.9%), Proteus species 15 (6.8%), Coagulase Negative Staphylococcus 7 (3.2%) [Table/Fig-2]. A 136 (82%) of bacterial isolates and 18 (50%) of fungal isolates were biofilm producers [Table/Fig-3]. Staphylococcus aureus was the strong biofilm producer, followed by Pseudomonasaeruginosa, Klebsiella species, and Acinetobacter baumannii [Table/Fig-4]. Gram-negative bacteria showed high sensitivity to piperacillin-tazobactam, meropenem, gram-positive cocci to vancomycin and linezolid [Table/Fig-5]. A 15 (10%) of isolates were MDR organisms, Among 15 MDROs 13 (86.7%) showed biofilm formation. The MDR isolates tested for colistin were found to be sensitive, had MIC values of ≤2. On molecular characterisation [Table/Fig-6], blaCTX-M, blaTEM, blaNDM-1, blaOXA-23, mecA genes were present in resistant biofilm-forming isolates. Gram staining images of Gram positive cocci [Table/Fig-7], Gram negative bacteria [Table/Fig-8] and Canida albicans are depicted in [Table/Fig-9]. Gel documentation image showing amplified gene products in bp detected using 100 bp ladder in Escherichia coli [Table/Fig-10], MRSA [Table/Fig-11], Pseudomonasaeruginosa [Table/Fig-12], Acinetobacter baumannii [Table/Fig-13], and Klebsiella pneumoniae [Table/Fig-14].
Co-morbidity and complications associated with diabetes mellitus (N=150).
| Complications | No. of patients (N=150) | Percentage |
|---|
| Anaemia | 135 | 90 |
| Neuropathy | 85 | 56.7 |
| Nephropathy | 54 | 36 |
| Peripheral vascular disease | 38 | 25.3 |
| Retinopathy | 26 | 17.3 |
| Cardiovascular disease | 15 | 10 |
| Hypertension | 40 | 26.7 |
Distribution of pathogens in diabetic ulcer (N=150).
| Group | Isolates | Number | Percentage |
|---|
| GPC (54 isolates)25% | Staphylococcus aureus | 33 | 15.2 |
| Enterococcus faecalis | 14 | 6.4 |
| Staphylococcus epidermidis | 7 | 3.2 |
| GNB (112 isolates)52% | Pseudomonas aeruginosa | 37 | 17.1 |
| Acinetobacter baumannii | 15 | 6.9 |
| Klebsiella pneumoniae | 19 | 8.8 |
| Klebsiella oxytoca | 3 | 1.4 |
| Escherichia coli | 17 | 7.9 |
| Citrobacter freundii | 4 | 1.8 |
| Enterobacter species | 2 | 0.9 |
| Proteus vulgaris | 4 | 1.8 |
| Proteus mirabilis | 11 | 5.0 |
| Fungal (38 isolates)18% | Candida albicans | 20 | 9.2 |
| Candida tropicalis | 5 | 2.3 |
| Candida parapsilosis | 9 | 4.1 |
| Candida glabrata | 2 | 0.9 |
| Aspergillus flavus | 1 | 0.4 |
| Aspergillus niger | 1 | 0.4 |
| Anaerobes (12 isolates) 5% | Peptostreptococcus anaerobius | 10 | 4.6 |
| Bacteroides | 2 | 0.9 |
| Total | | 216 | |
GPC: Gram positive cocci; GNB: Gram negative bacilli
Distribution of biofilm producers in diabetic ulcer (N=150).
| Organism | Biofilm | Non biofilm | Total |
|---|
| Staphylococcus aureus | 28 | 5 | 33 |
| Enterococcus faecalis | 10 | 4 | 14 |
| Staphylococcus epidermidis | 4 | 3 | 7 |
| Pseudomonas aeruginosa | 32 | 5 | 37 |
| Acinetobacter baumannii | 12 | 3 | 15 |
| Klebsiella species | 20 | 2 | 22 |
| Escherichia coli | 14 | 3 | 17 |
| Proteus species | 12 | 3 | 15 |
| Citrobacter freundii | 3 | 1 | 4 |
| Enterobacter species | 1 | 1 | 2 |
| Percentage | 136 (82%) | 30 (18%) | 166 (100%) |
| Fungal Isolates |
| Candida albicans | 10 | 10 | 20 |
| Candida parapsilosis | 5 | 4 | 9 |
| Candida tropicalis | 3 | 2 | 5 |
| Candida glabrata | 0 | 2 | 2 |
| Percentage | 18 (50%) | 18 (50%) | 36 (100%) |
Distribution of biofilm in bacterial pathogens (N=136).
| Organism | Weak biofilm | Moderate biofilm | Strong biofilm |
|---|
| Staphylococcus aureus | 14 | 4 | 10 |
| Enterococcus faecalis | 6 | 2 | 2 |
| Staphylococcus epidermidis | 3 | 1 | - |
| Pseudomonas aeruginosa | 15 | 9 | 8 |
| Klebsiella species | 11 | 2 | 7 |
| Escherichia coli | 9 | 3 | 2 |
| Proteus species | 10 | - | 2 |
| Acinetobacter baumannii | 6 | - | 6 |
| Citrobacter freundii | 2 | - | 1 |
| Enterobacter species | 1 | - | - |
| Fungal Isolates (N=18) |
| Candida albicans | 6 | 3 | 1 |
| Candida parapsilosis | 3 | 2 | - |
| Candida tropicalis | 2 | - | 1 |
| Candida glabrata | - | - | - |
Distribution of antimicrobial susceptibility pattern of pathogens in diabetic wounds.
| OragnismsAntibiotics | S. aureus (MSSA) | S. aureus (MRSA) | S. epidermidis | Enterococci | E. coli | Klebsiella species | Proteus species | Citrobacter species | Enterobacter species | Pseudomonas aeruginosa | Acinetobacter species |
|---|
| No.Isolated | N=19 | N=14 | N=7 | N=14 | N=17(ESBL-2) | N=22(ESBL-6) | N=15 | N=4 | N=2 | N=37 | N=15 |
| Ampicillin | NA | NA | NA | 8 | 2 | NA | NA | NA | NA | NA | NA |
| NA | NA | NA | 57% | 12% | NA | NA | NA | NA | NA | NA |
| Amikacin | NA | NA | NA | NA | 13 | 14 | 9 | 3 | 2 | 23 | 6 |
| NA | NA | NA | NA | 76% | 64% | 60% | 75% | 100% | 63% | 40% |
| Cefotaxime | NA | NA | NA | NA | 4 | 6 | NA | 1 | 1 | NA | NA |
| NA | NA | NA | NA | 24% | 28% | NA | 25% | 50% | NA | NA |
| Ceftazidime | NA | NA | NA | NA | NA | NA | 0 | NA | NA | 12 | 3 |
| NA | NA | NA | NA | NA | NA | 0% | NA | NA | 33% | 20% |
| Cefoxitin | 19 | 0 | 5 | NA | NA | NA | NA | NA | NA | NA | NA |
| 100% | 0% | 71% | NA | NA | NA | NA | NA | NA | NA | NA |
| Ciprofloxacin | NA | NA | NA | NA | 3 | 5 | 4 | 0 | 1 | 13 | 4 |
| NA | NA | NA | NA | 18% | 23% | 27% | 0 | 50% | 36% | 27% |
| Cotrimoxazole | 11 | 5 | 5 | NA | 5 | 6 | 5 | 0 | 1 | NA | 4 |
| 57% | 36% | 71% | NA | 29% | 28% | 34% | 0 | 50% | NA | 27% |
| Erythromycin | 13 | 5 | 5 | NA | NA | NA | NA | NA | NA | NA | NA |
| 68% | 36% | 71% | NA | NA | NA | NA | NA | NA | NA | NA |
| Gentamicin | NA | NA | NA | NA | 9 | 12 | 8 | 2 | 1 | 18 | 3 |
| NA | NA | NA | NA | 53% | 54% | 54% | 50% | 50% | 49% | 20% |
| High level gentamicin | NA | NA | NA | 10 | NA | NA | NA | NA | NA | NA | NA |
| NA | NA | NA | 71% | NA | NA | NA | NA | NA | NA | NA |
| Linezolid | 19 | 14 | 7 | 14 | NA | NA | NA | NA | NA | NA | NA |
| 100% | 100% | 100% | 100% | NA | NA | NA | NA | NA | NA | NA |
| Meropenem | NA | NA | NA | NA | 14 | 17 | 12 | 4 | 2 | 28 | 11 |
| NA | NA | NA | NA | 88% | 78% | 80% | 100% | 100% | 76% | 74% |
| Penicillin | 3 | 0 | 2 | 5 | NA | NA | NA | NA | NA | NA | NA |
| 16% | 0% | 29% | 36% | NA | NA | NA | NA | NA | NA | NA |
| Piperacillin- tazobactam | NA | NA | NA | NA | 15 | 16 | 11 | 4 | 2 | 29 | 11 |
| NA | NA | NA | NA | 88% | 72% | 74% | 100% | 100% | 79% | 74% |
| Tetracycline | 18 | 9 | 7 | NA | NA | NA | NA | NA | NA | NA | 9 |
| 94% | 64% | 100% | NA | NA | NA | NA | NA | NA | NA | 60% |
| Vancomycin | 19 | 14 | 7 | 14 | NA | NA | NA | NA | NA | NA | NA |
| 100% | 100% | 100% | 100% | NA | NA | NA | NA | NA | NA | NA |
Molecular characterisation of resistant genes by PCR.
| Resistant strains | Primers | Result |
|---|
| Staphylococcus aureus (5) | mecA | Positive |
| ESBL E.coli (1) | blaCTX-M | Positive |
| ESBL K.pneumoniae (2) | blaCTX-M, blaTEM | Positive |
| Pseudomonas aeruginosa (1) | blaNDM | Positive |
| Acinetobacter baumanii (1) | blaNDM-11, blaOXA-23 | Positive |
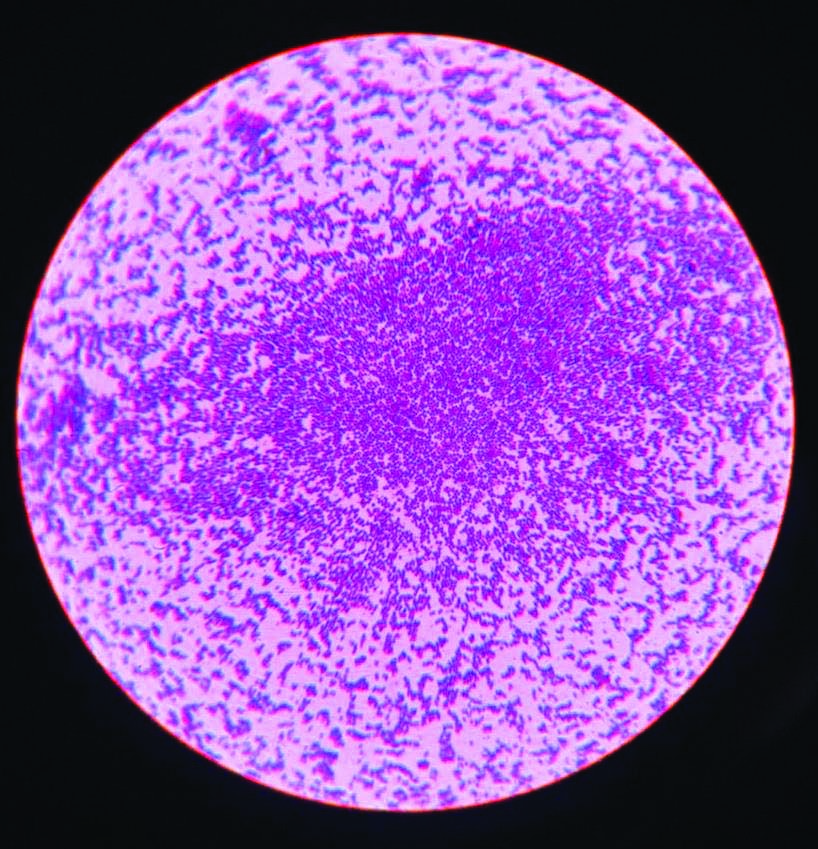
Gram stain: Gram positive cocci in clusters- Staphylococus aureus
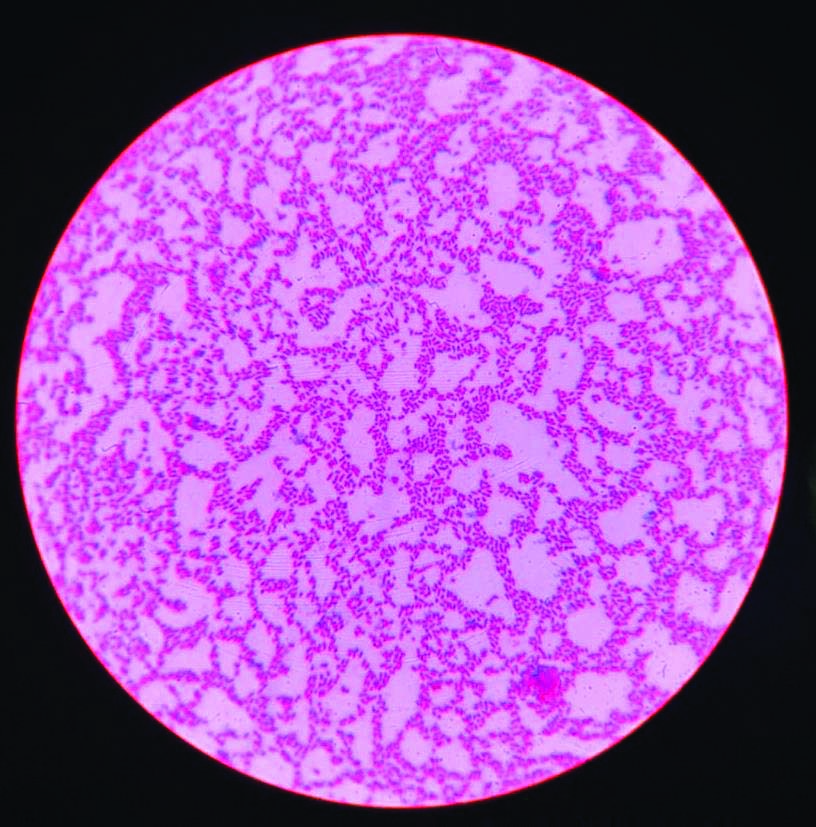
Gram stain: Gram negative bacilli- Klebsiella pneumoniae. Magnification 1000X.
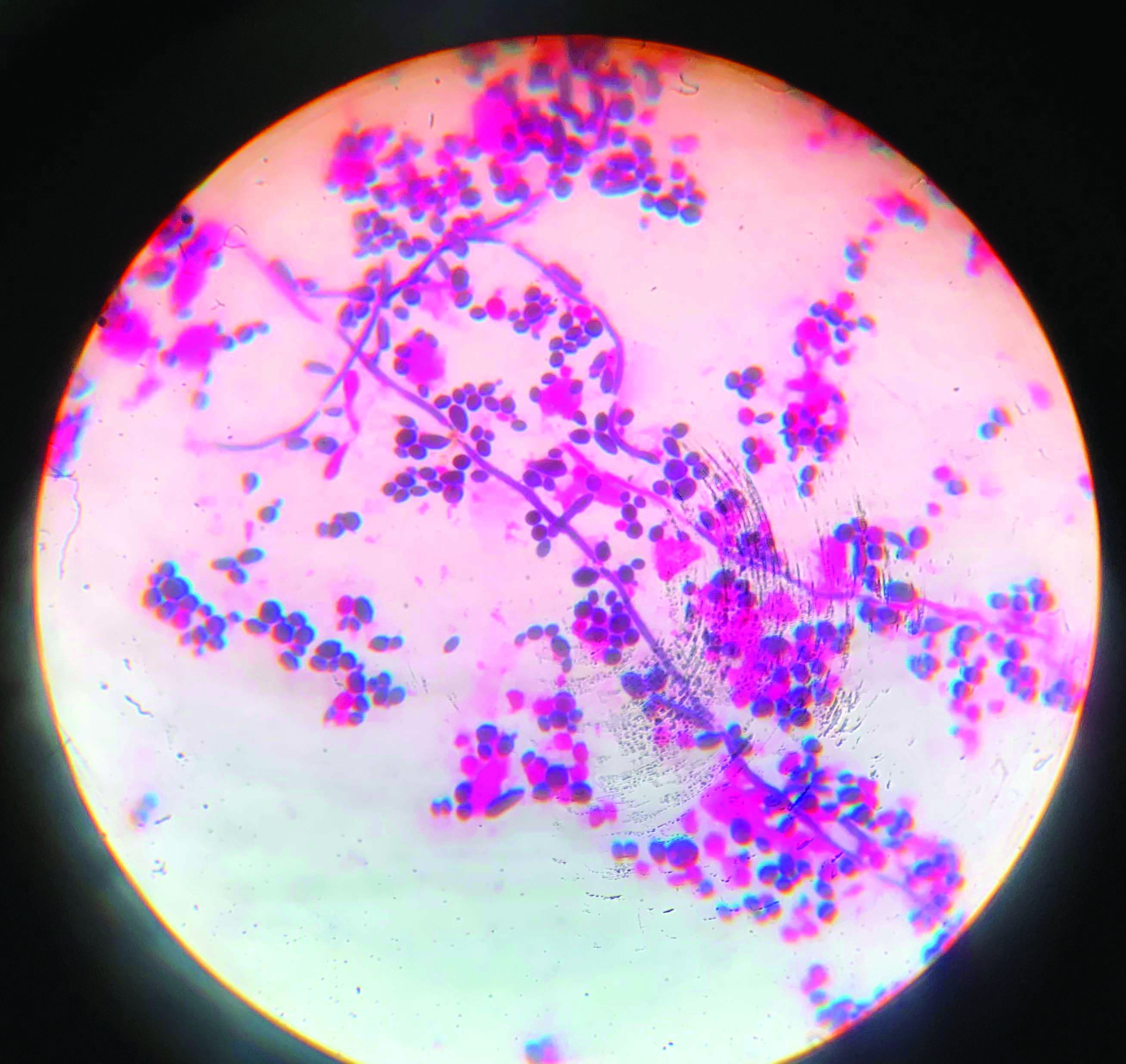
Gram stain- Candida albicans with pseudo hyphae, Magnification 1000X.
Detection of blaCTX-M Gene in E.coli.
Detection of mecA Gene in Methicillin-resistant S.aureus.
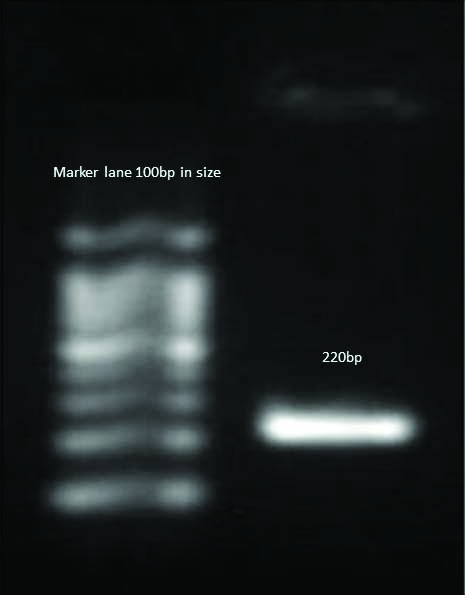
Detection of blaNDM-1 gene in P.aeruginosa.
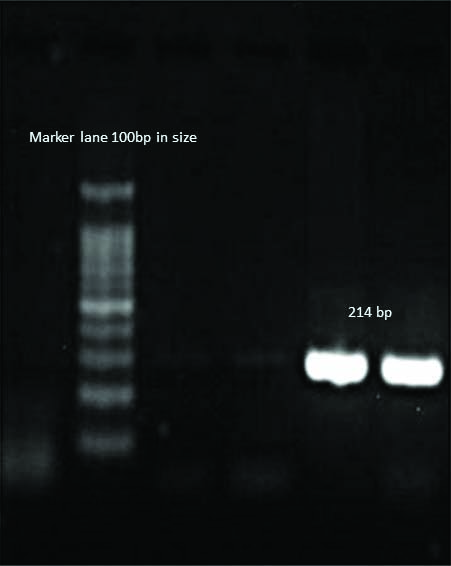
Detection of blaNDM-1, blaOXA-23 gene in A.baumannii;

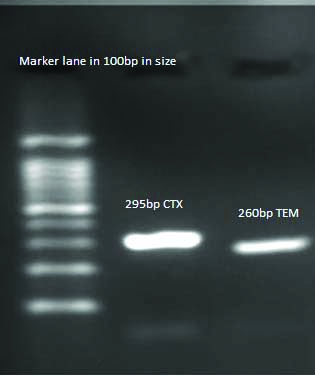
Detection of blaCTX-M, blaTEM Gene in K.penumoniae.
Discussion
Of 150 patients included in the study, 34.7% of patients were from the age group of 51-60 years; males were predominant (53.3%) than females (46.7%). Neuropathy (56.7%) was the commonest complication, followed by nephropathy (36%), and anaemia (90%) was the major co-morbidity associated with Diabetes Mellitus. Thus, early identification and correction of modifiable risk factors such as anaemia may slow the progression and improve patient survival. Ekpebegh CO et al., and Chuan F et al., also studied the association of anaemia and amputation in diabetic patients [12,13]. However, anaemia as an independent predictor of outcome is unknown.
A 216 isolates were detected from 150 ulcer specimens averaging 1.44 isolates per patient, showing 46% polymicrobial and 48% monomicrobial infection. A study was done by Ramakant P et al., (66%) has well documented the Polymicrobial nature of wounds [14]. Indeed, Trengrove NJ et al., had suggested that rather than the mere presence of specific microorganisms, the occurrence of polymicrobes in chronic wounds leads to a delay in wound healing [24]. In this study, gram-negative bacilli (52%) was the predominant pathogen, among them Pseudomonas aeruginosa (17.1%) being the common aetiological agent, followed by Klebsiella species (6.9%), Escherichia coli (7.9%), Proteus species (6.8%) and Acinetobacter baumannii (10%) in agreement with the study conducted by Gadepalli R et al., Kumar A et al., in India [25,26]. Gram-negative bacteria preponderance was due to the immunocompromised state of diabetes. Patients are highly susceptible to hospital-acquired infections either by environmental strain colonisation or following invasive surgical procedures. In this study, Staphylococcus aureus was predominantly isolated among the isolated gram positive cocci, with 42.4% isolates exhibiting Methicillin Resistance (MRSA). Similar studies were seen by Gadepalli R et al., (56%), Murali TS et al., (67%) [25,27]. The diversity of pathogens in different studies is influenced by the source of infection, type, and severity of infection, sample collection, use of antibiotics for treatment and geographical variations.
Peptostreptococcus (4.6%) and Bacteroides 0.9% were isolated, all from grade 3 ulcers. Studies have reported a higher percentage of anaerobic isolates (Smith K et al., 25%, Anyim O et al., 53%) [28,29]. Among fungal growth of 17.4%, Candida species were frequently isolated, predominant being Candida albicans (52.6%). Similar results were seen in Arun CS et al., (40%) [30].
In the present study, among the pathogens, 71% of isolates were biofilm producers. Pseudomonas (23.5%) was the predominant biofilm former, followed by Staphylococcus aureus (20.5%). The same observation was reported in Percival SL et al., 14 isolates were methicillin-resistant Staphylococcus aureus [31]. Of these, 10 of the isolates (72%) were strong biofilm producers. A study done by Gordon RJ and Lowy FD, had supported the biofilm-forming nature of Staphylococcus aureus [32]. In this study, 60% Candida tropicalis, 55% Candida parapsilosis, and 50% Candida albicans were biofilm producers against the corresponding non-biofilm Candida species. In similarity to the study by Deorukhkar SC et al., where Candida tropicalis (74%) exhibited higher biofilm-forming ability [33].
Antimicrobial susceptibility pattern gram negative bacilli showed high susceptibility to meropenem (95%), piperacillin-tazobactam (78%), amikacin (68%), tetracycline (60%), and high-level resistance to ampicillin (88%), ceftazidime (82%), ciprofloxacin (70%). This was in agreement with the findings of Rani V and Nithyalakshmi J, Banu A et al., [34,35]. Similarly, Halpati A et al., showed imipenem and piperacillin-tazobactam as the most effective drug against ESBL producing organisms [36]. The MRSA has a prevalence of 42.4%, which was similar to previous studies of Bansal E et al., (64.9%), Viswanathan V, MRSA exhibited resistance of 100% to penicillin G, 64% to erythromycin, 64% to cotrimoxazole [37,3]. All Staphylococcus aureus isolates were sensitive to linezolid (100%) and vancomycin (100%). In addition, all the MRSA isolates were vancomycin (100%) sensitive.
In present study, 10% of isolates were MDR. Among the MDR, 86.7% showed biofilm formation. It was similar to the study conducted by Swarna S et al., [38]. The MDR isolates tested for Colistin sensitivity by broth microdilution, 5 isolates showed MIC value of ≤0.5, 6 isolates had MIC value of 1 and 2 isolates had MIC value of 2 and all are found to be sensitive similar to Kumar A et al., (100% sensitive) [26].
Molecular analysis of resistant isolates by PCR detected blaCTX-M gene in ESBL producing Escherichia coli and blaCTX-M, blaTEM gene in ESBL producing Klebsiella species, mecA gene in Methicillin-resistant Staphylococcus aureus, blaNDM-1 gene in carbapenem-resistant Pseudomonas aeruginosa, and blaNDM-1, blaOXA-23 resistant gene in Acinetobacter baumannii.
Limitation(s)
Larger sample size would have provided accurate statistical tests to assess significance and correlation.
Conclusion(s)
Early identification and correction of modifiable risk factors such as anaemia may slow the progression and improve the patient survival in Diabetic foot syndrome. In chronic diabetic wound microbiome, fungi act as a niche to form intricate biofilms causing poor antibiotic penetration, adaptive stress responses, nutrient limitation, and slow growth. Nonetheless, the formation of persister cells contributes to drug resistance. Overuse and misuse of antibiotics can lead to the evolution of resistant strains. Antimicrobial resistance and the emergence of MDR organisms are a potential threat in the community too. This reflects the need for global strategies to control the emergence and spread of MDR pathogens.
GPC: Gram positive cocci; GNB: Gram negative bacilli