Gestational Diabetes Mellitus (GDM), a frequent medical complication of pregnancy, is defined as “any degree of glucose intolerance with onset or first recognition during pregnancy” [1,2]. This condition results in various adverse pregnancy outcomes such as macrosomia, still births, neonatal metabolic abnormalities, maternal diabetes etc., [3,4]. Maternal hyperglycaemia due to pre-existing diabetes or GDM, leads to foetal hyperinsulinaemia and diabetic foetopathy [5]. Complications in afflicted pregnant women include pre-eclampsia, caesarean section and increased risk of future diabetes [6-8].
Although OGTT is considered the gold standard diagnostic test for diagnosing GDM, it is very cumbersome and inconvenient for the patient, as it requires overnight fasting followed by multi-invasive 3-4 blood sample collections. The process of oral glucose loading itself leads to nausea and discomfort to the already fasting pregnant female. On the other hand, a single blood sample for HbA1c investigation can be collected irrespective of the fasting status and does not require multiple pricks and inconvenient glucose loading. The Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study has shown significant association between raised HbA1c levels and higher occurrence of adverse outcomes in pregnancy [9]. Hence, the present study was done with an aim to ascertain whether HbA1c can be used instead of OGTT for diagnosis of GDM.
Materials and Methods
The present study was a case-control study conducted for a period of one year from June 2012 to May 2013. In this study, 200 pregnant women in the age group of 20-40 years, from Gynaecology Out Patient Department (OPD) were selected using convenient sampling for the study after taking the informed consent. The study was conducted after ethical approval from the Institutional Ethical Committee vide letter No. FN/11/IEC/MAMC/2012/215, dated 28.5.12. The present study was according to Declaration of Helsinki.
Inclusion criteria: Pregnant women in 2nd and 3rd trimester from 20-40 years of age.
Exclusion criteria: Non pregnant women. Pregnant women with pre-existing diabetes. Patients suffering from anaemia, chronic renal and pancreatic diseases, diabetes mellitus, genetic disorders and haemoglobinopathies were excluded from the study.
The pregnant females were divided into two groups: Group 1 and Group 2, based on GDM positive and GDM negative status as per OGTT, the index test.
Each pregnant women underwent GCT with 50 g glucose, OGTT and HbA1c investigations. The sample for HbA1c was collected and analysed along with the first OGTT sample. OGTT was performed according to American Diabetes Association (ADA) guidelines with 100 g glucose load and threshold values of 95, 180, 155 and 140 mg/dL for fasting, 1 hour, 2 hour and 3 hour post glucose load were considered. Utilising 100 g OGTT as the index method, two or more values meeting or exceeding the threshold values were used to confirm GDM.
The glucose estimation was done by the Glucose oxidase (GOD) and Peroxidase (POD). (Reference value: 70-110 mg/dL) method on Beckman coulter DXC-800 Autoanalyser and HbA1c (Reference value: 4.5-5.7%) was done by High Performance Liquid Chromatography (HPLC) method on Biorad D-10 analyser.
Statistical Analysis
The data collected was analysed using Statistical Package for Social Sciences (SPSS) version 20.0 software. Student’s t-test was performed to compare the means and Receiver Operator Characteristic (ROC) curve and was plotted to detect sensitivity and specificity at various cut-offs of HbA1c.
Results
The patient demographics is shown [Table/Fig-1].
Mean and standard deviations of demographics of both Group 1 and 2.
| Variables | Group | N | Mean±SD |
|---|
| Age (years) | GDM | 100 | 26.92±3.73 |
| Non GDM | 100 | 25.51±4.14 |
| Weight (kg) | GDM | 100 | 61.52±11.91 |
| Non GDM | 100 | 53.05±11.03 |
| Height (m) | GDM | 100 | 1.51±0.07 |
| Non GDM | 100 | 1.52±0.07 |
| BMI (kg/m2) | GDM | 100 | 27.09±5.96 |
| Non GDM | 100 | 22.89±4.60 |
| GCT (mg/dL) | GDM | 100 | 165.76±27.68 |
| Non GDM | 100 | 139.07±25.42 |
| HbA1c (%) | GDM | 96 | 5.29±0.68 |
| Non GDM | 98 | 4.83±0.46 |
There was no statistically significant difference between the cases and controls between the demographics used for the study. The controls were not included in statistical analysis. The controls were used to categorise the groups involved in the study. Four patients from GDM and two from non GDM were excluded from the calculation as their samples could not be processed for HbA1c estimation.
The pregnant females were divided into two groups based upon the presence or absence of GDM confirmed by OGTT, the index test. [Table/Fig-1] shows that the mean age of pregnant females in Group 1 (GDM) was 26.92±3.73 years and the mean age of pregnant females in Group 2 (Non GDM) was 25.51±4.14 years. The mean weight in non pregnancy Group 1 was 61.52±11.9 kg whereas it is 53.05±11.03 kg in Group 2. The mean pre-pregnancy BMI was 27.09±5.96 kg/m2 in Group 1 and it was 22.89±4.60 kg/m2 in Group 2. The mean of GCT is also higher in Group 1 i.e., 165.76±27.68 mg/dL as compared to that of Group 2 which is 139.07±24.42 mg/dL. The mean HbA1c of Group 1 with GDM was 5.29±0.68% and mean HbA1c of Group 2 without GDM was 4.83±0.46%.
[Table/Fig-2] shows the ROC curve area for HbA1c to detect GDM in 2nd and 3rd trimester was 0.74 (95% CI 0.673-0.815). At the HbA1c cut-off of ≥5.85%, the sensitivity for diagnosing GDM was 18% and specificity was 70%. The sensitivity and specificity was 16.3% and 97% at HbA1c cut-off of ≥5.95%. The HbA1c cut-off ≥6.05% showed sensitivity and specificity of 16.3% and 98.1%, respectively. It was observed that as the HbA1c cut-off was lowered there was an improvement in the sensitivity and specificity in diagnosing GDM. This signified the fact that HbA1c levels during pregnancy are lower than that of non pregnant females, thus corroborating with lower glucose levels during pregnancy [10]. The area under the curve and the coordinates of the curve are presented in [Table/Fig-3,4], respectively.
ROC Curve showing specificity and sensitivity in GDM group (n=96).
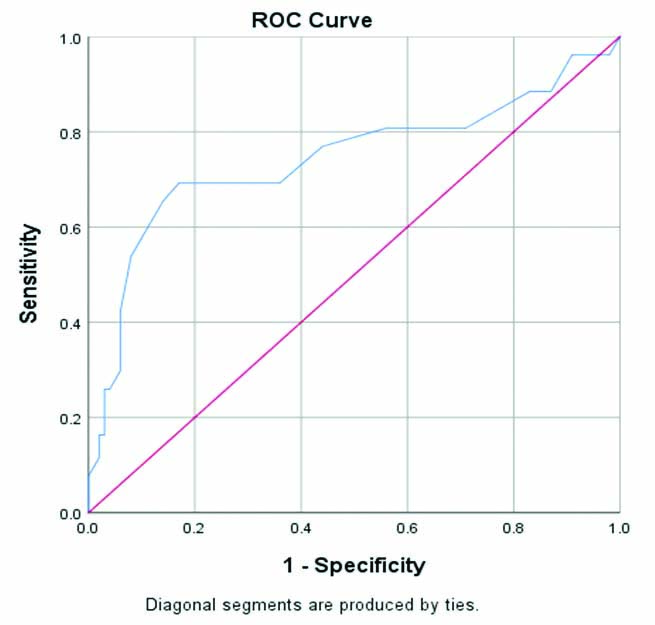
| Area | Std. errora | Asymptotic sig.b | Asymptotic 95% confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 0.744 | 0.036 | <0.001 | 0.673 | 0.815 |
Coordinates of the curve.
| Positive if greater than or equal toa | Sensitivity | Specificity |
|---|
| 3.1000 | 1.000 | 1.000 |
| 4.1500 | 0.962 | 0.980 |
| 4.2500 | 0.962 | 0.910 |
| 4.3500 | 0.885 | 0.870 |
| 4.4500 | 0.885 | 0.830 |
| 4.5500 | 0.808 | 0.710 |
| 4.6500 | 0.808 | 0.650 |
| 4.7500 | 0.808 | 0.560 |
| 4.8500 | 0.769 | 0.440 |
| 4.9500 | 0.692 | 0.360 |
| 5.0500 | 0.692 | 0.250 |
| 5.1500 | 0.692 | 0.170 |
| 5.2500 | 0.654 | 0.140 |
| 5.3500 | 0.538 | 0.080 |
| 5.4500 | 0.423 | 0.060 |
| 5.5500 | 0.298 | 0.060 |
| 5.6500 | 0.260 | 0.040 |
| 5.7500 | 0.260 | 0.030 |
| 5.8500 | 0.173 | 0.030 |
| 5.9500 | 0.163 | 0.030 |
| 6.0500 | 0.163 | 0.020 |
| 6.2000 | 0.154 | 0.020 |
| 6.5000 | 0.115 | 0.020 |
| 6.7500 | 0.077 | <0.001 |
| 7.8000 | <0.001 | <0.001 |
a: Under non parametric assumption
Discussion
This study was aimed at evaluating the accuracy of HbA1c as a diagnostic test for GDM. The mean HbA1c of Group 1 with GDM was 5.29±0.68% and mean HbA1c of Group 2 without GDM was 4.83±0.46%. The mean HbA1c of Group 1 was lower than the lower limit of reference range adopted by ADA for non pregnant adult. This observation emphasises the fact that HbA1c levels are lowered during pregnancy which corroborates with lowered glucose levels during pregnancy. Thus, the physiological decrease in HbA1c during normal pregnancy has to be considered as diagnostically and clinically important, when, setting the desirable levels of HbA1c in a pregnancy which is complicated with diabetes.
Studies by Mosca A et al., and Nielsen LR et al., have also reported lower HbA1c levels in pregnancy compared to non pregnant state [11,12]. According to them, the mean HbA1c in non pregnant women was 5.5±0.4%, whereas in the present study, the mean HbA1c in non GDM group was 4.83±0.46%. This can be explained by the fact that there was a fall in fasting plasma glucose levels due to utilisation of glucose by the foetus during pregnancy. As a result the erythrocytes are exposed to lower glucose concentration compared to non pregnant adults thus decreasing the degree of glycation [13,14].
In the present study, 100 g OGTT was performed in all the pregnant females and ROC curve analysis was used to scrutinise the performance of HbA1c in diagnosing GDM. The area under ROC curve value for HbA1c was 0.74 (95% CI 0.673-0.815). At the HbA1c cut-off of ≥5.85%, 18% of GDM patients were identified with specificity of 97%. A higher HbA1c cut-off of ≥5.95% showed sensitivity and specificity of 16.3% and 70%, respectively. In contrast, Rajput R et al., in their study reported sensitivity of 11.9% but a higher specificity of 97.1% at HbA1c cut-off of ≥5.95% [15]. In another study done by Renz PB et al., they have reported sensitivity and specificity of 20.7% and 97.1% at HbA1c cut-off of ≥ 5.9% [16].
The present study showed a better specificity of HbA1c at cut-off value of ≥5.85% as compared to ADA recommended cut-off of ≥5.7% for the diagnosis of prediabetes in non pregnant state. However, the HbA1c cut-off of ≥5.85% would have diagnosed only 18% of GDM patients previously confirmed with 100 g OGTT with reasonable specificity.
Limitation(s)
The small sample size was the limitation of the present study. Since lower levels of glucose are observed during pregnancy, the sensitivity and specificity of HbA1c at lower cut-offs should be explored with a larger population group.
Conclusion(s)
Although, the sensitivity and specificity of HbA1c in diagnosing GDM is not exactly superlative, still it can be used as a supplemental investigation with OGTT. Moreover, present study highlights the fact that there is a lack of fixed HbA1c cut-off for diagnosing GDM, as it is subjected to a number of dynamic variables during pregnancy. Thus, HbA1c can be used to supplement OGTT in diagnosing GDM.
a: Under non parametric assumption