Association of Motor Function and Neuroimaging in Cerebral Palsy: A Cross-sectional Study
Dhirendra Kumar Singh1, Nonica Laisram2, Amita Malik3, Vinay Kanaujia4, Suman Badhal5, Sakshi Jain6
1 Senior Resident, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, Delhi, India.
2 Principal Consultant and Ex. Additional Directorate General of Health Services, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, Delhi, India.
3 Professor and Consultant, Department of Radiodiagnosis, VMMC and Safdarjung Hospital, Delhi, India.
4 Assistant Professor, Department of Physical Medicine and Rehabilitation, SUHMMH Medical College, Saharanpur, Uttar Pradesh, India.
5 Professor, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, Delhi, India.
6 Assistant Professor, Department of Physical Medicine and Rehabilitation, Hamdard Institute of Medical Sciences and Research and HAHC Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sakshi Jain, 274-C, J and K Pocket, Dilshad Garden, Delhi-110095, India.
E-mail: jsakshi32@yahoo.com
Introduction
Cerebral Palsy (CP) is one of the most common causes of disability in children. Neuroimaging is useful in determining structural or functional relationships in children with CP. It provides an opportunity to link various CP types to the localisation of the brain maldevelopment or lesion.
Aim
To study association of motor function and brain structure on neuroimaging Magnetic Resonance Imaging (MRI) in CP children.
Materials and Methods
This cross-sectional observational study was conducted in a tertiary care hospital from August 2015 to December 2016. A total of 50 diagnosed cases of CP were included after satisfying inclusion and exclusion criteria. After detailed history and clinical examination, MRI of brain was advised. All parameters were assessed in terms of Gross Motor Functional Classification System (GMFCS), Manual Ability Classification System (MACS) and MRI Grading. Categorical variables were presented as numbers and percentage and association was checked using Chi-square test or Fischer’s-exact test. A p-value of <0.05 was considered statistically significant.
Results
Patients with CP in the study ranged from 2-12 years with mean age of 5.43 (±2.58) years. Out of 50 cases, 30 (60%) were in the age group of 2-5 years, 17 (34%) were in the age group of 6-10 years and three (6%) were in the age group of 11-12 years. Out of 50 enrolled cases, maximum cases were having GMFCS level 3 (n=14) and MACS level 2 (n=15). There was significant positive association (p-value <0.05) between GMFCS level and grading of MRI. Similar significant association was observed for analysis of association of MACS level and MRI Grading.
Conclusion
The present study highlighted that there was a significant association between extent and type of brain lesion and motor functions (GMFCS and MACS levels). Type and extent of brain lesion helps clinician to understand prognosis of functional motor outcome in CP children.
Brain lesion, Gross motor functional classification system, Magnetic resonance imaging, Manual ability classification system
Introduction
The Cerebral Palsy (CP) is one of the most common physical disability in children with a prevalence rate of 2 to 2.5 per 1,000 live births [1]. It has been defined as “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain [2]. The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, and behaviour, by epilepsy, and by secondary musculoskeletal problems” [2].
Injury to the immature brain resulting in CP can occur in the prenatal, perinatal, or postnatal periods in both term and preterm infants. It is now thought that most cases of CP stem from an injury which occurred in the prenatal period [3,4]. Common prenatal causes include Toxoplasmosis, Rubella, Cytomegalovirus, Herpes Simplex, other (TORCH) or other infections, intrauterine stroke (ischemic and haemorrhagic), toxemia, and genetic malformation. The perinatal events like placental abruption, cord prolapse, uterine rupture, or similar processes resulting in birth asphyxia can lead to CP. Fortunately, these aetiologies are relatively rare, and it has been shown in a previous study that an interruption of oxygen supply to the foetus does not account for most cases of CP [4]. Postanatal causes of CP include cerebrovascular insults (ischaemic or haemorrhagic), Central Nervous System (CNS) infection, acquired head injury, kernicterus, anoxia, hydrocephalus etc. The aetiology or pathophysiology of the brain damage varies with gestational age, and thus influences the CP subtype and associated movement disorder [4].
Growth and differentiation events predominate during the third trimester and continue into postnatal life. Disturbances in brain development during this time result in lesions that are somewhat different from those caused by earlier insults or developmental abnormalities. The Peri Ventricular-White Matter (PV-WM) is particularly damaged in the early third trimester, however towards the end of the third trimester grey matter appears to be more affected [5].
Since the greater use of neuroimaging in the evaluation of children with congenital or early-onset neurological impairments, our understanding of the aetiologies of CP in the patient has increased significantly. Improved neuroimaging has allowed researchers to link different types of CP to the location of a brain maldevelopment or lesion. According to the American Academy of Neurology all children with a suspected diagnosis of CP should undergo neuroimaging, with Magnetic Resonance Imaging (MRI) being preferred over Computed Tomography (CT) because it provides precise localisation of the lesion [6]. This helps to understand and recognise the functional limitation and function of various rehabilitation interventions towards their improvement. Improved neuroimaging has provided an opportunity to link various CP types to the localisation of the brain maldevelopment or lesion. Neuroimaging is currently useful in determining structural or functional relationships in children with CP [7,8]. On literature search, we could not find any similar study with similar parameters done in India. Hence the present study aimed to study motor function in CP and its association with brain structure on neuroimaging (MRI) and grading of MRI.
Materials and Methods
This study was a single centred cross-sectional observational study, conducted in the Department of Physical Medicine and Rehabilitation (PMR) at Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India, from August 2015 to December 2016 after taking approval from Institutional Ethical Committee (IEC/Oct/2015). Patients were screened from the Outpatient Department of PMR. Baseline demographic data and detailed history were recorded, and physical and neurological examination was carried out. The CP was diagnosed according to a standard clinical definition as stated above [2]. Cases were enrolled in the study after satisfying the inclusion criteria and exclusion criteria.
Inclusion and Exclusion criteria: Diagnosed cases of CP within age group of 2-12 years were included and any case with congenital anomalies of upper and lower limb, musculoskeletal abnormalities of upper and lower limb, or with metallic implants were excluded.
Sample size calculation: On the basis of previous study, prevalence of CP was 2 to 2.5 per 1000 live birth [1]. Taking this value as reference, the minimum required sample size with 2% margin of error and 5% level of significance was 24 patients. To reduce margin of error, total sample size taken was 50.
Informed consent was taken from parents or guardians. Selected patients were advised neuroimaging (MRI).
For the motor function all the patients were assessed using Gross Motor Function Classification System (GMFCS) scale [9] and MACS [10].
a) GMFCS LEVELS: I-Walks without limitations, II-Walks with limitations, III-Walks using a hand-held mobility device, IV-Self-mobility with limitations; May use powered mobility, V-Transported in a manual wheelchair.
b) MACS LEVELS: I-Handles objects easily and successfully, II-Handles most objects but with somewhat reduced quality and/or speed of achievement, III-Handles objects with difficulty; needs help to prepare and/or modify activities, IV-Handles a limited selection of easily managed objects in adapted situations, V-Does not handle objects and has severely limited ability to perform even simple actions.
Magnetic Resonance Imaging (MRI) was done with 1.5 T Philips InteraAchieva MR scanner. T1weighted, T2 weighted, T2 Fluid Attenuation Inversion Recovery (FLAIR) sequences were obtained in axial plane. T2 weighted imaging were done in sagittal and coronal plane. The MRI was analysed by the Radiologist for lesion changes and grading. The lesions were described as:
Hypomyelination,
Cerebral atrophy,
Malformation,
Enlargement of lateral ventricle,
Periventricular leukomalacia,
Porencephaly,
Border zone infarction,
Thin corpus callosum,
Cerebellar atrophy.
For grading of MRI [11] we had used the scoring system of sub-component like:
Size of lateral ventricles
Normal size of both ventricles
Unilateral enlargement or bilateral mild Enlargement
Bilateral severe enlargement
White Matter (WM) abnormal signal intensity
Normal WM or only focal involvement of PV-WM
Diffuse involvement of PV-WM in both hemispheres or involvement of Subcortical White Matter (SC-WM) in one hemisphere
Involvement of SC-WM in both hemispheres
WM reduction
Not reduced
Reduction of PV-WM in both hemispheres or of deep WM diffusely in one hemisphere
Reduction of deep WM diffusely in both hemispheres
Cysts
No cysts,
Small cysts (n<3) bilateral in PV regions or unilateral cystic lesion (small or large),
Bilateral multiple cysts (small or large) involving PV regions and/or deep WM
Size of subarachnoid space
No enlargement
Bilateral diffuse mild enlargement or severe enlargement only in one hemisphere
Diffuse severe enlargement in both hemispheres
Corpus callosum
Normal or thinning involving the posterior body
Thinning involving the total body
Diffuse thinning
Cortical grey matter
No cortical abnormalities
Unilateral cortical abnormalities
Bilateral cortical abnormalities
Grade-1 was considered normal having score 7-11, grade-2 was mild having score 12-16 and grade-3 was severe with score of 17-21.
Statistical Analysis
In the statistical analysis, categorical variables were presented in number and percentage (%) and continuous variables were presented as mean±SD and median. Normality of data was tested by Kolmogorov-Smirnov test. If the normality was rejected then non-parametric test were used. Qualitative variables were correlated using Chi-Square test/Fisher’s-exact test. A p-value of ≤0.05 was considered statistically significant. The data were entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
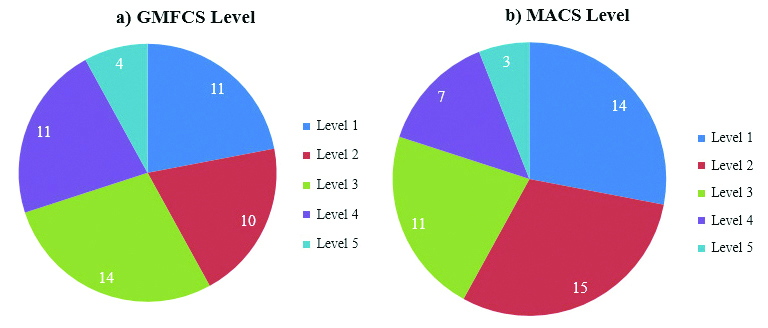
Patients with CP in the study ranged from 2-12 years with mean age of 5.43 (±2.58) years. Out of 50 cases, 30 (60%) were in the age group of 2-5 years, 17 (34%) were in the age group of 6-10 years and 3 (6%) were in the age group of 11-12 years. Thirty two (64%) were males and 18 (36%) were females. The Male: Female ratio was 1.78:1. Maximum number of cases 14 (28%) were of GMFCS level 3 and least number of cases were of GMFCS level 5 in 4 (8%) cases. Maximum number of cases, 15 (30%) were of MACS 2 and least number of cases, 3 (6%) were of MACS level 5 [Table/Fig-1]. The most common type of brain lesion present was type E (Periventricular leukomalacia) seen in 45 (90%) cases [Table/Fig-2].
Distribution of Cases according to a) GMFCS level; and b) MACS level (N=50).
| Type of brain lesion | No. of cases | Percentage |
|---|
| A (Hypomyelination) | 0 | 0 |
| B (Cerebral atophy) | 4 | 8% |
| C (Malformation) | 0 | 0 |
| D (Enlargement of lateral ventricle) | 34 | 68% |
| E (Periventricular leukomalacia) | 45 | 90% |
| F (Porencephaly) | 6 | 12% |
| G (Border zone infarction) | 24 | 48% |
| H (Thin corpus callosum) | 31 | 62% |
| I (Cerebellar atrophy) | 1 | 2% |
| No lesion | 4 | 8% |
Type F (Porencephaly) was present mostly in GMFCS level 3 and in few cases of GMFCS levels 4 and 5 which was statistically significant (p-value=0.04) [Table/Fig-3]. Type F (Porencephaly) brain lesion was seen in MACS level 3, 4 and 5 with p-value=0.018. Type G (Border zone infarction) and H (Thin corpus callosum) were seen in all MACS level [Table/Fig-4].
Association of motor function (GMFCS Level) and type of brain lesion.
| Type of lesion | GMFCS (n,%) | Total | p-value* |
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| A | 0 | 0 | 0 | 0 | 0 | 0 | - |
| B | 0 | 1 (10%) | 1 (7.14%) | 1 (9.09%) | 1 (25%) | 4 (8%) | 0.624 |
| C | 0 | 0 | 0 | 0 | 0 | 0 | - |
| D | 7 (63.64%) | 6 (60%) | 9 (64.29%) | 8 (72.73%) | 4 (100%) | 34 (68%) | 0.649 |
| E | 10 (90.91%) | 8 (80%) | 12 (85.71%) | 11 (100%) | 4 (100%) | 45 (90%) | 0.546 |
| F | 0 | 0 | 1 (7.14%) | 4 (36.36%) | 1 (25%) | 6 (12%) | 0.04 |
| G | 4 (36.36%) | 4 (40%) | 6 (42.86%) | 7 (63.64%) | 3 (75%) | 24 (48%) | 0.517 |
| H | 5 (45.45%) | 6 (60%) | 8 (57.14%) | 8 (72.73%) | 4 (100%) | 31 (62%) | 0.352 |
| I | 0 | 0 | 1 (7.14%) | 0 | 0 | 1 (2%) | 0.623 |
| No lesion | 0 | 2 (20%) | 2 (14.29%) | 0 | 0 | 4 (8%) | 0.29 |
| Total** | 11 (100%) | 10 (100%) | 14 (100%) | 11 (100%) | 4 (100%) | 50 (100%) | - |
*Statistical tests used-Fischer’s-exact test; **Total number of subjects can have more than one type of lesion, A p-value of ≤0.05 was considered statistically significant
Association of motor function (MACS Level) and type of brain lesion.
| Type of lesion | MACS (n,%) | Total | p-value* |
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| A | 0 | 0 | 0 | 0 | 0 | 0 | - |
| B | 0 | 0 | 2 (18.18%) | 1 (14.29%) | 1 (33.33%) | 4 (8%) | 0.133 |
| C | 0 | 0 | 0 | 0 | 0 | 0 | - |
| D | 8 (57.14%) | 9 (60%) | 9 (81.82%) | 5 (71.43%) | 3 (100%) | 34 (68%) | 0.461 |
| E | 11 (78.57%) | 14 (93.33%) | 10 (90.91%) | 7 (100%) | 3 (100%) | 45 (90%) | 0.503 |
| F | 0 | 0 | 2 (18.18%) | 3 (42.86%) | 1 (33.33%) | 6 (12%) | 0.018 |
| G | 3 (21.43%) | 8 (53.33%) | 4 (36.36%) | 6 (85.71%) | 3 (100%) | 24 (48%) | 0.018 |
| H | 7 (50%) | 8 (53.33%) | 7 (63.64%) | 6 (85.71%) | 3 (100%) | 31 (62%) | 0.302 |
| I | 0 | 0 | 0 | 1 (14.29%) | 0 | 1 (2%) | 0.18 |
| No lesion | 2 (14.29%) | 1 (6.67%) | 1 (9.09%) | 0 | 0 | 4 (8%) | 0.795 |
| Total** | 14 (100%) | 15 (100%) | 11 (100%) | 7 (100%) | 3 (100%) | 50 (100%) | - |
*Statistical tests used-Fischer’s-exact test; **Total number of subjects can have more than one type of lesion, A p-value of ≤0.05 was considered statistically significant
Eleven (35.48%) patients in GMFCS level 1 had brain MRI grade 1 (normal) which was statistically significant (p-value=0.003). In GMFCS 4 predominant MRI grade was 2 (mild) which is statistically significant finding (p-value=0.013) [Table/Fig-5]. In MACS level 1 and 2 majority of cases were of MRI grade 1 and in MACS level 4 and 5 all the cases were of MRI grade 2 only [Table/Fig-6].
Association of motor function (GMFCS level) and brain MRI grading.
| Grading of MRI (n,%) | Total | p-value* |
|---|
| 1 | 2 | 3 |
|---|
| GMFCS | 1 | 11 (35.48%) | 0 | 0 | 11 (22%) | 0.003 |
| 2 | 7 (22.58%) | 3 (15.79%) | 0 | 10 (20%) | 0.722 |
| 3 | 9 (29.03%) | 5 (26.32%) | 0 | 14 (28%) | 0.836 |
| 4 | 3 (9.68%) | 8 (42.11%) | 0 | 11 (22%) | 0.013 |
| 5 | 1 (3.23%) | 3 (15.79%) | 0 | 4 (8%) | 0.147 |
| Total | 31 (100%) | 19 (100%) | 0 | 50 (100%) | - |
*Statistical tests used-Chi-square test, A p-value of ≤0.05 was considered statistically significant
Association of motor function (MACS Level) and brain MRI grading.
| Grading of MRI (n,%) | Total | p-value* |
|---|
| 1 | 2 | 3 |
|---|
| MACS | 1 | 13 (92.86%) | 1 (7.14%) | 0 | 14 (28%) | 0.008 |
| 2 | 13 (86.67%) | 2 (13.33%) | 0 | 15 (30%) | 0.026 |
| 3 | 5 (45.45%) | 6 (54.55%) | 0 | 11 (22%) | 0.201 |
| 4 | 0 | 7 (100%) | 0 | 7 (14%) | 0.001 |
| 5 | 0 | 3 (100%) | 0 | 3 (6%) | 0.049 |
| Total | 31 (100%) | 19 (100%) | 0 | 50 (100%) | - |
*Statistical tests used-Chi-square test, A p-value of ≤0.05 was considered statistically significant
Discussion
Cerebral palsy is a disorder that is most noticeably characterised by a motor disorder causing physical disability in human development, mainly in areas of body movement and posture. Study of motor status and its severity is vital for optimal therapy and rehabilitation. Motor function is affected by different type and extent of brain lesion [12].
Age distribution of patients in study population was in range of 2-12 years, with mean age of 5.43 (±2.58) years. Maximum number was in age group of 2-5 years. Among study population gender ratio was observed to be 1.78:1(M:F) showing male predominance which was comparable to a study done by Pharaoh PO et al., which shows male to female ratio of 1.4:1 with hemiplegic children, 1.2:1 for diplegic children and 1.5:1 for quadriplegic children [13].
In our study we observed most the patients belong to GMFCS class 1 and class 2 which corresponds to previous study [14,15]. Similarly most of the patients were of MACS level 1 and level 2 which also corresponds to study done by Himmelmann K et al., in 186 children. They found that Forty per cent of the children were in level 1 of the MACS and 19% were in level 2 [16].
In the present study, it was found that type E (Periventricular leukomalacia) brain lesion was most common lesion. It was observed consistently in all GMFCS level 1 (90.91%), 2 (80%), 3 (85.71%), 4 (100%) and 5 (100%) cases. Type D (Enlargement of lateral ventricle), type H (Thin corpus callosum) and type G (Border zone infarction) were next most common types of brain lesions. Two (20%) cases in GMFCS level 2 and 2 (14.29%) cases in GMFCS level 3 did not show any lesion on MRI brain. Numata Y et al., in their study on 86 patients found that 36 cases had normal brain MRI finding in all GMFCS levels. Fifty cases had abnormal MRI finding, type E (Periventricular leukomalacia) was found in 12 (14%), type D (Enlargement of lateral ventricle) in 12 (14%), type F (Porencephaly) 6 (7%), type A (Hypomyelination) five (5.8%), type B (Cerebral atrophy) four (4.7%), type C (Malformation) 3 (3.5%), type G (Border zone infarction) three (3.5%), type I (Cerebellar atrophy) 2 (2.3%), type H (Thin corpus callosum) 1(1.1%) and two cases had unclassified lesion [17].
It was found that in GMFCS level 1 only MRI grade 1 (normal) was seen, in GMFCS level 2 and 3 predominant MRI grade was 1 (normal) and in GMFCS level 4 and 5 predominant MRI grade was 2 (mild). With increasing GMFCS levels, MRI grade was also increasing. A Study by Robinson MN et al., has shown the similar results. However, MRI grade 3 lesions were not seen in any cases included in the present study [12].
Like GMFCS, in MACS level 1and 2 predominant MRI grade was 1 (p-value=0.008, 0.026), in MACS level 3 predominant MRI grade was 2 (p-value=0.201) and in MACS level 4 and 5 only MRI grade 2 lesion was seen (p-value=0.001, 0.049). There was direct association of MACS level with MRI grading. However, MRI grade 3 lesions were not seen in any MACS level. Our results are comparable with finding of earlier report by Kwong KL et al., who analysed the MRI of brain in 122 children with spastic CP and found that MRI findings for patients with spastic CP were closely related to types of CP and gestation at birth [18].
Limitation(s)
As this study was a time bound study on a limited number of cases, further studies are recommended with bigger sample size and longer time duration.
Conclusion(s)
The present study highlighted that there was association between type and extent of brain lesion and type of CP and motor functions (GMFCS and MACS levels). Type and extent of brain lesion helps clinician to understand functional motor outcome and application of rehabilitation strategies in CP rehabilitation. However, brain lesion is not present in MRI in some cases. Hence, proper clinical evaluation and analysis along with MRI findings are recommended in diagnosis and planning optimal rehabilitation interventions in CP.
*Statistical tests used-Fischer’s-exact test; **Total number of subjects can have more than one type of lesion, A p-value of ≤0.05 was considered statistically significant
*Statistical tests used-Fischer’s-exact test; **Total number of subjects can have more than one type of lesion, A p-value of ≤0.05 was considered statistically significant
*Statistical tests used-Chi-square test, A p-value of ≤0.05 was considered statistically significant
*Statistical tests used-Chi-square test, A p-value of ≤0.05 was considered statistically significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 14, 2021
Manual Googling: Jun 29, 2021
iThenticate Software: Jul 23, 2021 (22%)
[1]. Stanley F, Blair E, Alberman E, Cerebral Palsies: Epidemiology and Casual Pathways 2000 LondonMac Keith Press [Google Scholar]
[2]. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, A report: The definition and classification of cerebral palsy AprilDev Med Child Neurol 2007 49:08-14.10.1111/j.1469-8749.2007.tb12610.x [Google Scholar] [CrossRef]
[3]. Nelson KB, Grether JK, Causes of cerebral palsyCurr Opin Pediatr 1999 11:487-91.10.1097/00008480-199912000-0000210590904 [Google Scholar] [CrossRef] [PubMed]
[4]. Mukherjee S, Garbler-Spira DJ, Cerebral palsy. In: Braddom RL, editorPhysical Medicine and RehabilitationNew DelhiElsevier:1243-67. [Google Scholar]
[5]. Boyd RN, Jordan R, Pareezer L, Moodie A, Finn C, Luther B, Australian Cerebral Palsy Child Study: Protocol of a prospective population based study of motor and brain development of preschool aged children with cerebral palsyBMC Neurol 2013 13:5710.1186/1471-2377-13-5723758951 [Google Scholar] [CrossRef] [PubMed]
[6]. Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: Diagnostic assessment of the child with cerebral palsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology SocietyNeurology 2004 62(6):851-63.10.1212/01.WNL.0000117981.35364.1B15037681 [Google Scholar] [CrossRef] [PubMed]
[7]. Shapiro B, Cerebral palsy: A reconceptualization of the spectrumJ Pediatr 2004 145:03-07.10.1016/j.jpeds.2004.05.01415292880 [Google Scholar] [CrossRef] [PubMed]
[8]. Msall M, Limperopoulos C, Park J, Neuroimaging and cerebral palsy in childrenMinerva Pediatr 2009 61:415-24. [Google Scholar]
[9]. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B, Development and validation of a gross motor function classification system for children with cerebral palsyDev Med Child Neurol 1997 39:214-23.10.1111/j.1469-8749.1997.tb07414.x9183258 [Google Scholar] [CrossRef] [PubMed]
[10]. Eliasson AC, Krumlinde SL, Rösblad B, Beckung E, Arner M, Öhrvall AM, The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliabilityDev Med Child Neurol 2006 48:549-54.10.1017/S001216220600116216780622 [Google Scholar] [CrossRef] [PubMed]
[11]. Cioni G, Di Paco MC, Bertuccelli B, Paolicelli PB, Canapicchi R, MRI findings and sensorimotor development in infants with bilateral spastic cerebral palsyBrain Dev 1997 19(4):245-53.10.1016/S0387-7604(97)00569-X [Google Scholar] [CrossRef]
[12]. Robinson MN, Peake LJ, Ditchfield MR, Reid SM, Lanigan A, Reddihough DS, Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsyDev Med Child Neurol 2009 51:39-45.10.1111/j.1469-8749.2008.03127.x19018841 [Google Scholar] [CrossRef] [PubMed]
[13]. Pharoah PO, Cooke T, Rosenbloom L, Cooke RW, Effects of birth weight, gestational age, and maternal obstetric history on birth prevalence of cerebral palsyArch Dis Child 1987 62(10):1035-40.10.1136/adc.62.10.10353674922 [Google Scholar] [CrossRef] [PubMed]
[14]. Benedict RE, Patz J, Maenner MJ, Arneson CL, Yeargin-Allsopp M, Doernberg NS, Feasibility and reliability of classifying gross motor function among children with cerebral palsy using population-based record surveillancePaediatr Perinat Epidemiol 2011 25(1):88-96.10.1111/j.1365-3016.2010.01164.x21133973 [Google Scholar] [CrossRef] [PubMed]
[15]. Andersen GL, Irgens LM, Haagaas I, Skranes JS, Meberg AE, Vik T, Cerebral palsy in Norway: Prevalence, subtypes and severityEur J Paediatr Neurol 2008 12(1):04-13.10.1016/j.ejpn.2007.05.00117574886 [Google Scholar] [CrossRef] [PubMed]
[16]. Himmelmann K, Uvebrant P, Function and neuroimaging in cerebral palsy: A population-based studyDev Med Child Neurol 2011 53(6):516-21.10.1111/j.1469-8749.2011.03932.x21574988 [Google Scholar] [CrossRef] [PubMed]
[17]. Numata Y, Onuma A, Kobayashi Y, Sato I, Tanaka S, Kobayashi S, Brain magnetic resonance imaging and motor and intellectual functioning in 86 patients born at term with spastic diplegiaDev Med Child Neurol 2013 55:167-72.10.1111/dmcn.1201323121133 [Google Scholar] [CrossRef] [PubMed]
[18]. Kwong KL, Wong YC, Fong CM, Wong SN, So KT, Magnetic resonance imaging in 122 children with spastic cerebral palsyPediatr Neurol 2004 31(3):172-76.10.1016/j.pediatrneurol.2004.02.00515351015 [Google Scholar] [CrossRef] [PubMed]