The Supraglottic Airway Devices (SAD) is being increasingly used in anaesthesia [1] and nearly half of the General Anaesthesia (GA) cases are being done using SADs [2]. But insertion conditions for SADs are not always ideal with the standard induction protocol [3]. I-Gel is a second generation SAD has an advantage of no inflation of cuff. Its soft, non inflatable cuff fits snugly to create an anatomical seal (pharyngeal, laryngeal and perilaryngeal structures) onto the perilaryngeal framework [4]. The seal created is sufficient for both spontaneously breathing patients and those with intermittent positive pressure ventilation [5].
Nevertheless, at the standard doses of opioids and propofol, insertion conditions of SAD are not always ideal. The attenuation of pharyngo-laryngeal reflexes may not be sufficient and the patient can have coughing, gagging and laryngospasm during insertion attempts and occasionally insertion can be difficult. Nebulised lignocaine is already proven to reduce discomfort by nasopharyngeal instrumentation [6], awake flexible bronchoscopy [7] and endotracheal intubation [8], also been used for postoperative sore throat [9], oesophago-gastroduodenoscopy [10], pressor response [11], and emergence from sevoflurane [12].
Administration of lignocaine topically to the airway before induction of anaesthesia may obtund the airway reflex responses and render induction smoother. Topical Lignocaine has been tried to improve the insertion conditions of SAD [3]. As topical spray can be uncomfortable in awake patients and can itself stimulate laryngospasm in lightly anaesthetised patients, it was hypothesised that nebulised lignocaine can improve the insertion condition and first attempt insertion of SAD (I-Gel in our study), by providing topical anaesthesia of airway in patients undergoing small duration surgeries under GA.
Hence, this present study was conducted to assess the efficacy of lignocaine nebulisation for I-gel insertion conditions in terms of the frequency of optimal insertion conditions and haemodynamic changes observed during and after the insertion of I-gel.
Materials and Methods
This randomised controlled trial was conducted in 90 patients after Institutional Ethical Committee approval and written informed consent, for one year from January 2019 to January 2020 in Karnataka Institute of Medical sciences, Hubli, Karnataka, India.
Inclusion criteria: Patients aged between 18 to 45 years of ASA grade I and II, of either sex, posted for elective short surgical procedures (one to two hours) under GA were included.
Exclusion criteria: Smokers, obese (Body Mass Index >30 kg/m2), pregnant patients, patients with suspected difficult airway (Mallampatti grade 3 or 4) [13], patients with hypersensitivity to the study drugs, patients with significant cardiovascular, pulmonary, liver disease and patients with gastroesophageal reflux or hiatal hernia were excluded from the study.
Sample size calculation: Based on a study of Laryngeal Mask Airway (LMA) insertion with propofol and topical lignocaine and taking successful insertion rate at the first attempt as the primary outcome [3], for power of 80%, sample size of 43 was required. Considering a possible dropout, we took a sample size of 45 in each group.
One of the researcher enrolled the patients and enrolled patients were divided into two groups of 45 patients each, as per computer generated random numbers. Assignment of participants was done by second researcher, allocation concealment was done by opaque sealed envelopes. I-gel size was standardized based in weight and manufacturer’s recommendation, size 3 I-gel for 30-60 kg and size 4 I-gel for 50-90 kg [14]. Patients and principal investigator were blinded in this study.
All patients were subjected to preoperative screening including thorough history, physical examination and investigations like complete haemogram, renal function test and Electrocardiogram (ECG). Standard fasting protocols were followed (6 hours for solid food and 2 hours for clear liquid). All participants received tablet Diazepam 10 mg on the night before the surgery. On the day of surgery, patients were nebulised and nebulisation was done by anaesthesiologist not involved in the study. After securing intravenous access, lignocaine group (Group L) patients received 5 mL of lignocaine 4% nebulisation, and control group (Group D) patients received 5 mL of normal saline nebulisation five minutes prior to anaesthesia. Then patients were shifted to Operation Theatre (OT), infusion of Ringer Lactate started. Routine standard monitoring, Pulse Oximeter (SPO2), Non Invasive Blood Pressure (NIBP), capnography and ECG were recorded.
Procedure
All patients were preoxygenated with 100% oxygen for three minutes via mask gently placed over face and premedicated with with Injection Glycopyrrolate 0.01 mg/kg, Injection Fentanyl 2 mcg/kg and Injection Midazolam 0.05 mg/kg given intravenously (i.v.). Patient were induced with Injection propofol 2 mg/kg i.v. slowly and observed for loss of eyelash reflex and no response to vocal commands, as the end point of induction. Before insertion, I-gel was lubricated with water based jelly. I-gel insertion was done by standard technique, by an experienced anaesthesiologist (who has done more than 25 I-gel insertions) [15].
Appropriate placement of I-gel was confirmed by observing movement of chest wall, auscultation and a square wave end-tidal CO2 (EtCO2) waveform during spontaneous or mechanical ventilation. During insertion our primary objective “Frequency of optimal insertion conditions” and side-effects were recorded. The condition for I-gel insertion described as “optimal” when there were no coughing, gagging, laryngospasm or body movements and when the I-gel inserted successfully on the first attempt.
In both the groups, time taken for I-gel insertion was taken from time of stopping the face mask ventilation to appearance of a square wave capnography trace after I-gel placement [15] and incidence of apnoea (absence of spontaneous breathing for >30 seconds) [16] was noted. During I-gel insertion, if it was impossible to open the patient’s mouth, further attempts were preceded by propofol 0.5 mg/kg and also when the airway reflexes prevented the I-gel insertion or when gross body movement which required restraining the patient was noted, an additional dose of propofol 0.5 mg/kg was given [6]. But insertion conditions were assessed only for first attempt. Any failure of insertion in both groups (after three attempts) was managed by injection. Succinylcholine i.v. and endotracheal intubation.
After successful placement of I-gel and confirmation of air entry in the lungs, patient was maintained on spontaneous ventilation with nitrous oxide (N2O): Oxygen (O2) and sevoflurane. Haemodynamic monitoring like heart rate, SBP and DBP were noted before, immediately after and five minutes after the insertion of I-gel. At end of procedure I-gel was removed when patients fulfilled the criteria of I-gel removal (spontaneous eye opening, obeying verbal commands). Any blood stains over I-gel were observed and followed-up for postoperative sore throat.
Statistical Analysis
Data was entered into Microsoft excel data sheet and analysed using Statistical Package for the Social Sciences (SPSS 22.0) version software. Student’s t-test was used for the parametric data (blood pressure measurements, apnoea time, insertion time and time to loss of consciousness). Chi-square test was used for non parametric measurements. The p-value <0.05 was considered significant.
Results
Ninety patients were selected for the study among which three patients were dropped from the study (one patient intubated intraoperatively, one bilateral abdominal tubectomy case was under spinal anaesthesia and one fibroadenoma case was performed under local anaesthesia). A total of 43 patients were in group lignocaine nebulisation (Group L) and 44 in the normal saline nebulisation group (Group D). Flowchart of the study population is given in [Table/Fig-1] (Attrition rate was 3.3%).
CONSORT flowchart of study population.
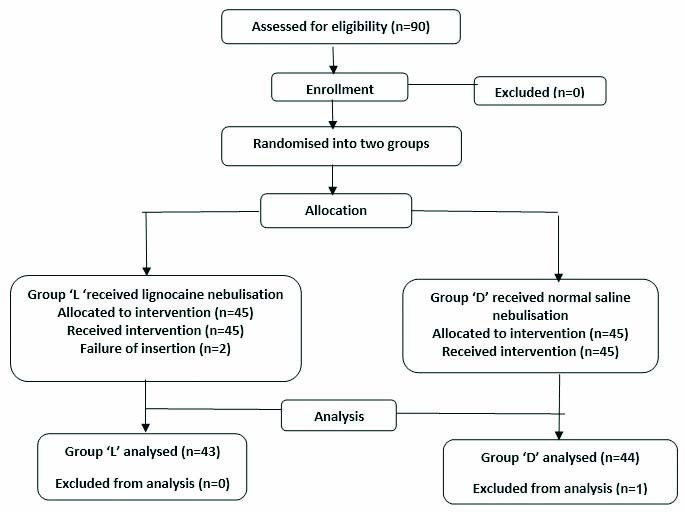
Both groups were comparable in terms of age, gender, height, weight, BMI, ASA grading as shown in [Table/Fig-2]. The [Table/Fig-3] compares the optimal insertion of the I-gel between the two groups. The group L had 38 (88.4%) optimal insertions, 5 (11.6%) did not. In the group D, there were 35 (79.5%) optimal insertions and 9 (20.5%) were not optimal. The p-value between the two groups was >0.05 which is statistically not significant.
Demographic profiles of both groups.
| Variables | Lignocaine group (L) (n=43) Mean±SD | Normal saline group (D) (n=44) Mean±SD | p-value |
|---|
| Age (years) | 28.8±6.3 | 29.0±5.1 | 0.865 |
| Gender (M:F) | 14:29 | 8:36 | 0.123 |
| Height (cm) | 160.3±8.4 | 159.2±6.3 | 0.517 |
| Weight (kg) | 59.8±9.3 | 56.9±6.6 | 0.107 |
| BMI (kg/m2) | 23.2±2.6 | 22.4±1.7 | 0.109 |
| ASA grading class (I/II) | 38/5 | 39/5 | 0.969 |
Chi-square test -p-value <0.05 was considered significant
Comparison of optimal insertion in two groups.
| Optimal insertion | Group L (n=43) | Group D (n=44) | p-value |
|---|
| Yes | 38 (88.4%) | 35 (79.5%) | 0.263 |
| No | 5 (11.6%) | 9 (20.5%) |
Chi-square test -p-value <0.05 was considered significant
The [Table/Fig-4] compares the successful first attempt insertion of the I-gel between the two groups. Statistical analysis using Chi-square test shows that in the group L 97.7% successful first attempt insertions, 2.3% were not. In the group D, there were 90.9% successful first attempt insertions and 9.1% were not. The p-value of comparison of first attempt insertion between the two study groups was >0.05 which was statistically not significant. The [Table/Fig-5] compares the additional propofol required between the two groups. Statistical analysis using Chi-square test showed that there was no statistically significant difference between the two groups (p>0.05).
Comparison of number of attempts between two groups (n=87).
| Successful first attempt | Group L (n=43) | Group D (n=44) | p-value |
|---|
| Yes | 42 (97.7%) | 40 (90.9%) | 0.175 |
| No | 1 (2.3%) | 4 (9.1%) |
Chi-square test -p-value <0.05 was considered significant
Comparison of need for propofol between two groups (n=87).
| Additional propofol requirement | Group L (n=43) | Group D (n=44) | p-value |
|---|
| Not required | 37 (86.0%) | 37 (84.1%) | 0.722 |
| 20 mg | 2 (4.7%) | 3 (6.8%) |
| 30 mg | 3 (7.0%) | 4 (9.1%) |
| 40 mg | 1 (2.3%) | 0 |
Chi-square test -p-value <0.05 was considered significant
[Table/Fig-6] shows the distribution of SBP and DBP at different time point between the two groups. The changes in SBP and DBP between the two groups were comparable.
Distribution of systolic and diastolic pressures among two groups at various time points.
| Parameter | Group L | Group D | p-value | Group L | Group D | p-value |
|---|
| Diastolic pressure (mm of Hg) | Systolic pressure (mm of Hg) |
|---|
| Before I-gel insertion | 73±8.8 | 72.0±7.1 | 0.579 | 119.6±12.1 | 117±28.7 | 0.329 |
| Immediately after insertion | 69.8±8.9 | 70.6±6.7 | 0.608 | 113.1±12.2 | 114.5±8.8 | 0.525 |
| 5 min after insertion | 66.2±7.1 | 68.7±6.6 | 0.083 | 103.6±18.8 | 106.6±17.3 | 0.436 |
| Postoperative | 67.1±6.5 | 68.5±5.8 | 0.315 | 110.7±11.2 | 112.5±7.7 | 0.365 |
Unpaired Student’s test -p-value <0.05 was considered significant
[Table/Fig-7] shows the distribution of heart rate at different time point between the two groups. The changes in heart rate between two groups were comparable.
Comparison of Heart rate between two groups at various time points (n=87).
| Parameter heart rate (bpm) | Group L (n=43) | Group D (n=44) | p-value |
|---|
| Before I-gel insertion | 89.6±13.2 | 84.4±11.8 | 0.0581 |
| Immediately after insertion | 91.8±11.2 | 83.6±9.5 | 0.001 |
| 5 min after insertion | 92.2±10.5 | 83.6±9.5 | 0.056 |
| Postoperative | 86.4±9.6 | 81.0±8.4 | 0.006 |
Unpaired Student’s test -p-value <0.05 was considered significant
The [Table/Fig-8] shows comparison of time to loss of consciousness, insertion time and apnoea time between the two study groups. Time to loss of consciousness (p<0.0001) is statistically significant between two groups. The group L has apnoea time of 6 minutes with SD of 2.2 minutes and group D has apnoea time of 4.3 minutes with SD of 1.3 minutes and p-value <0.0001 which is statistically significant.
Comparison of time to loss of consciousness, insertion time and apnoea time between the two study groups (n=87).
| Parameter (in minutes) | Group L (mean±SD*) | Group D (mean±SD) | p-value |
|---|
| Time to loss of consciousness | 28.3±11.2 | 37.1±10.1 | <0.0001 |
| Insertion time | 8.3±2.3 | 8.6±1.8 | 0.5127 |
| Apnoea time (minutes) | 6.0±2.2 | 4.3±1.3 | <0.0001 |
Unpaired Student’s test -p-value <0.05 was considered significant
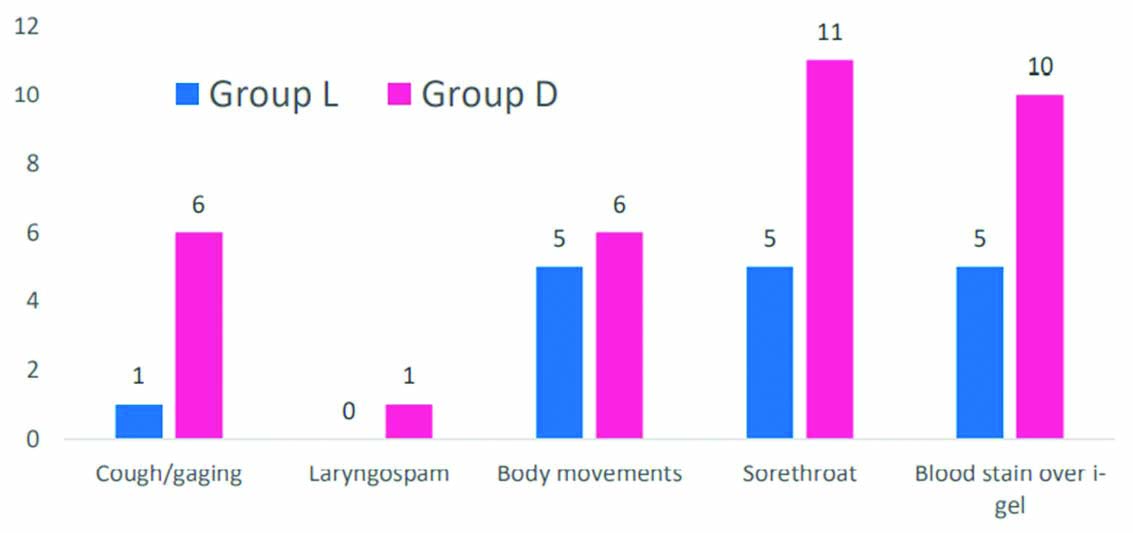
The [Table/Fig-9] represents the incidence of adverse effects among the two groups. The adverse events were less in lignocaine group.
Adverse events among the two groups during insertion and removal of I-gel.
Discussion
The present study showed that lignocaine nebulisation (in addition to standard induction protocol) did not offer any statistically significant advantage in improving the insertion conditions of I-gel in patients undergoing minor surgeries.
Topical, especially nebulised lignocaine, has been studied for airway instrumentation procedures, and has been proved beneficial in improving the tolerance of techniques in these patients. Nebulised lignocaine from commonly used nebulisers, produces effective oropharyngeal anaesthesia and is usually well tolerated by the patients [17,18].
The demographic profile of the current study was comparable to similar other studies [3,15,16] and did not show any statistical significance with respect to age, gender, height weight, BMI, ASA grading and type of surgery (p-value >0.05, statistically not significant). The age group of 18-45 years was chosen, as there is a possibility of suppression of protective airway reflexes and its consequences like aspiration, in the elderly age group. First attempt insertion with I-gel in most of studies showed to range between 82% to 100%, and the overall success rate is around 93% to 100% [1,19]. The results in our study were in consistent with this range of values.
The first attempt success rate of insertion in lignocaine group was 97.7% whereas it was 90.9% in the control group. The data shows probability of first attempt insertion is more with lignocaine group, but it is statistically not significant (p-value >0.05). Lee MC et al., study have found to have 100% success rate in first attempt insertion following lignocaine nebulisation along with remifentanil [20] where in study by Dabas P, Rasquinha JM et al., quoted 92.5% first attempt insertion with standard induction with propofol and fentanyl [21]. Similar results were noted in Wharton NM et al., (82.5%), and Helmy AM et al., [22,23]. In support of nebulised lignocaine Rao MH et al., have demonstrated that topical (gargling) lignocaine group had 83.3% success rate of first attempt insertion [24].
Optimal insertion was established in 88.4% of cases in study group, whereas 79.5% in the control group, in our current study. Even through more proportion of optimal condition was seen in our study group, it was statistically proved not significant (p-value >0.05). Ahmed S et al., found that, topical lignocaine showed excellent insertion condition (success rate of 83.3%) for LMA [25]. Lee MC et al., have demonstrated that topical lignocaine with remifentanil will allow successful insertion of LMA in healthy awake patients [20]. Our study results are in contrast to Changchein CF et al., who showed that topical lignocaine with propofol (2 mg/kg) provides statistically significant improvement in the optimal insertion condition, compared to high doses of propofol (3 mg/kg) [3].
Time for I-gel insertion varies in wide range, in various studies (between 4 to 15 seconds). Mean time for insertion in our study was found to be 8.3±2.3 seconds in study group and 8.6±1.8 seconds in control group. The p-value is >0.05 and hence, duration between two groups is statistically insignificant. Results in our study were comparable to findings of study conducted by Saracoglu KT et al., where the average time for insertion was 7.97±2.18 seconds [26]. In studies by Luthra A et al., (25.45 seconds) Muneer MN et al., (14±3.7 seconds) have found longer time for insertion [16,27]. Our study noted that time for loss of consciousness explained by loss of eyelash reflex, in lignocaine group (28.3±11.2 seconds) is statistically significant when compared to control group (37.1±10.1 seconds). Hence, lignocaine group is superior to control group in terms of time for loss of consciousness. In support of our results, the time for loss of consciousness was significantly less in group receiving lignocaine nebulisation before LMA insertion, in the study conducted by Changchein CF et al., [3]. This early loss of consciousness could not be ascertained due to lack of supportive literature. Prolonged apnoea time in lignocaine group noted in our study, could be explained by topical lignocaine anaesthesia which probably abolished the stimulation by the presence of I-gel. Mean apnoea time in lignocaine group (6.0±2.2 minutes) was significantly prolonged than control group. Our result is in contrast to Changchein CF et al., who found less incidence of apnoea in group with topical lignocaine and propofol probably because of their induction devoid of opioids, whereas we have used fentanyl for induction in both the groups [3]. Haemodynamic parameters were monitored continuously, and were compared between two groups with respect to baseline, immediately after insertion of I-gel, 5th minute and postoperatively.
Even though haemodynamic responses are not exaggerated after insertion of I-gel, there are conflicting data in literature regarding responses. Comparison of haemodynamic changes in our study, between two groups revealed more proportionate fall in both SBP and DBP, immediately after insertion of the I-gel and at 5th minute, but were statistically insignificant (p-value 0.05). This trend of decrease in blood pressure after induction has also been quoted by Ankur D et al., who reported fall in pressure soon after induction with standard technique [28]. Fall of mean arterial pressures has also been seen with lignocaine nebulisation, when it was used for attenuating presser response as reported by Kumar A et al., [29]. Mean heart rate in lignocaine group in our study was comparatively high immediately after insertion of I-gel, this could be explained by compensatory increase in heart rate for the fall in blood pressure after induction, which was observed in the current study, but results are statistically not significant. Our study results were in contrast to Changchein CF et al., where they found reduced heart rate after insertion of LMA in the topical lignocaine group [3].
We have observed for adverse events during I-gel insertion in our study, which includes coughing/gagging, body movements and laryngospasm. In our study, these were observed, comparatively more in the control group, but are statistically not significant. Changchein CF et al., study showed to have lesser incidence of coughing/gaging (7%) and body movement (17%) in lignocaine group [3].
Pharyngeal morbidities observed in our study are blood stain over I-gel and sore throat. According to literature, incidence of blood stain over I-gel is in between 4 to 13% [30]. In our study, the blood stain was reported to be less than these values- 11.6% in the lignocaine group and still higher in control group (22.7%). In a study by Muneer MN et al., they reported markedly higher incidence of blood stain over I-gel (29.3%) [27], whereas in study by Kim H et al., the incidence of blood stains on I-gel was quite low (6%) [31].
Incidence of sore throat in our study was 11.6% in lignocaine group and 25% in study group. According to literature, incidence of sore throat with I-gel insertion ranges between 5 to 17%. Whereas higher incidence of sore throat (32.5%) was found in the Helmy AM et al., study [23]. Dhanda A et al., study has reported lesser incidence of both blood stain (6.67%) and sore throat (6.67%) [28]. The results regarding blood stain over I-gel and postoperative sore throat in our study were statistically insignificant.
Over all propofol consumption in both the groups, were comparable even though we had anticipated lesser consumption of propofol in the study group because of topical anaesthesia.
Nebulised lignocaine is easily available, can easily to be delivered, has immediate and short duration of action, well tolerated by patients, less side-effect and minimal systemic absorption [8]. Although we did not assess the plasma lignocaine levels following nebulisation, dosage in our study group has been well within the safe limits. According to British Thoracic society, topical lignocaine up to 8.2 mg/kg has been recommended for safe use [32]. Parkes SB et al., have shown safe plasma level of lignocaine with nebulisation up to 4 mg/kg [33]. Authors have not observed symptoms or signs of lignocaine toxicity in our study.
Lignocaine nebulisation to improve the insertion conditions of I-gel is a novel idea that has not been tried before. Our trial was a prospective randomised double blinded study fulfilling all requirements of research study. Lignocaine is a relatively inexpensive drug available in the operating rooms.
Limitation(s)
The intraoperative seal pressure and mean airway pressures were not monitored. We have not included the pain, recovery and sedation score anywhere in our study and duration of topical anaesthesia was not assessed postoperatively.
Conclusion(s)
From the present study, it can be concluded that, pre-induction lignocaine nebulisation does not improve insertion conditions of I-gel statistically. Even though, there was less incidence of adverse events with lignocaine group, it was statistically insignificant.
Chi-square test -p-value <0.05 was considered significant
Chi-square test -p-value <0.05 was considered significant
Chi-square test -p-value <0.05 was considered significant
Chi-square test -p-value <0.05 was considered significant
Unpaired Student’s test -p-value <0.05 was considered significant
Unpaired Student’s test -p-value <0.05 was considered significant
Unpaired Student’s test -p-value <0.05 was considered significant