Case Report
A 61-year-old man presented to the Odontostomatology Department with a burning sensation and bleeding in the left mandibular region for more than two weeks in November 2017. The patient’s medical history revealed that he was not immunosuppressed and never underwent organ transplants, did not suffer from chronic or autoimmune inflammatory systemic diseases and that he had never undergone radio-chemotherapy. On physical examination of the head and neck region, no palpable lymph nodes were present. Blood tests for HIV, hepatitis B and C were also negative. Personal habits revealed a 20-year tobacco use (about one pack every day) and 20-year alcoholic abuse. He denied the use of illegal drugs. Tobacco and alcohol have been discontinued five years prior to the visit.
From January to April 2017 the patient had intraoral recurrent abscesses in the left posterior mandibular region. The previous dentist extracted the second lower left molar due to recurrent abscesses, assuming a periodontal disease and the patient was treated with repeated administration of antibiotics (amoxicillin and clavulanic acid, 2 g/per day, per one week) without a complete remission. Preoperatively, the patient was placed on antibiotic prophylaxis and had an uneventful extraction. The medical history of the patient revealed osteopenic vertebral collapse at L2 for which he has been treated with Clodronate disodium since August 2017. After seven months, an exophytic lesion appeared in the postextraction site without any other associated symptoms.
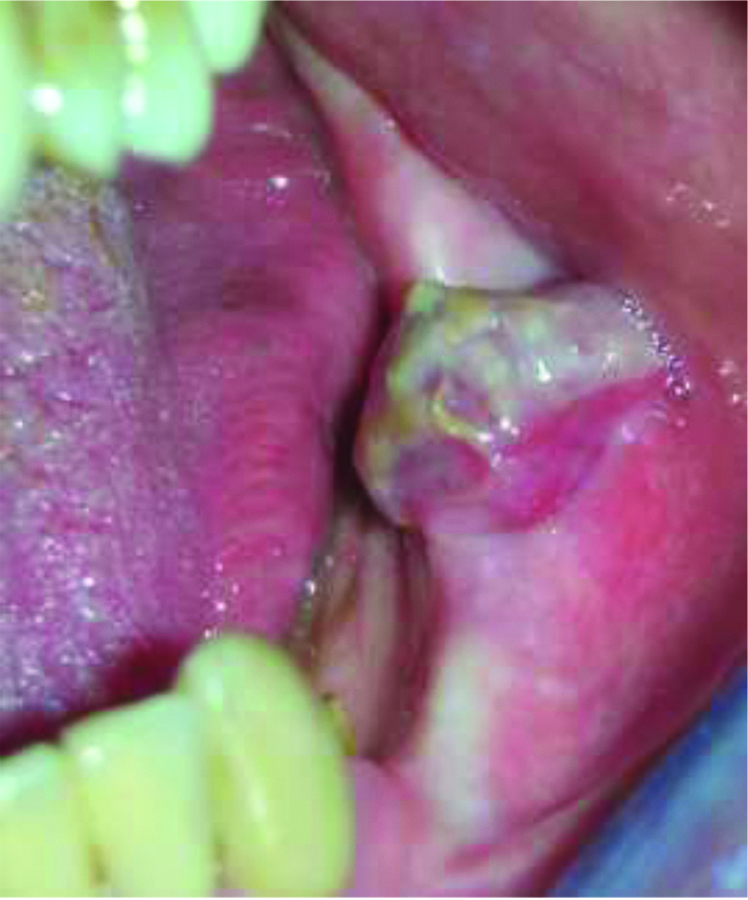
Intraoral examination revealed an exophytic lesion of approximately 2.5×4×3.5 cm in size, firm, covered by a whitish pseudomembrane, with spontaneous bleeding along the alveolus in relation to the edentulous space of previous extraction [Table/Fig-1].
An exophytic lesion in the left posterior mandibular area.
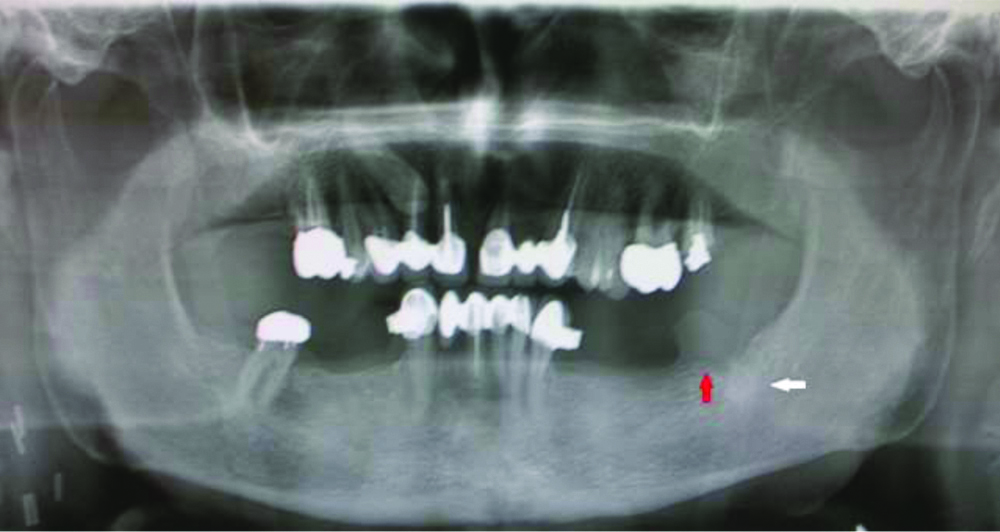
The lesion was primarily located within the soft tissues and there was no medullary involvement in the radiographic examination [Table/Fig-2].
Orthopantomogram (OPG) revealed incomplete healing of post extractive alveolus below the lesion. The bone corticals were thickened without discontinuities (see red arrow). There was an area of osteocondensation under the alveolus with no medullary infiltration or involvement of the inferior alveolar nerve (see white arrow).
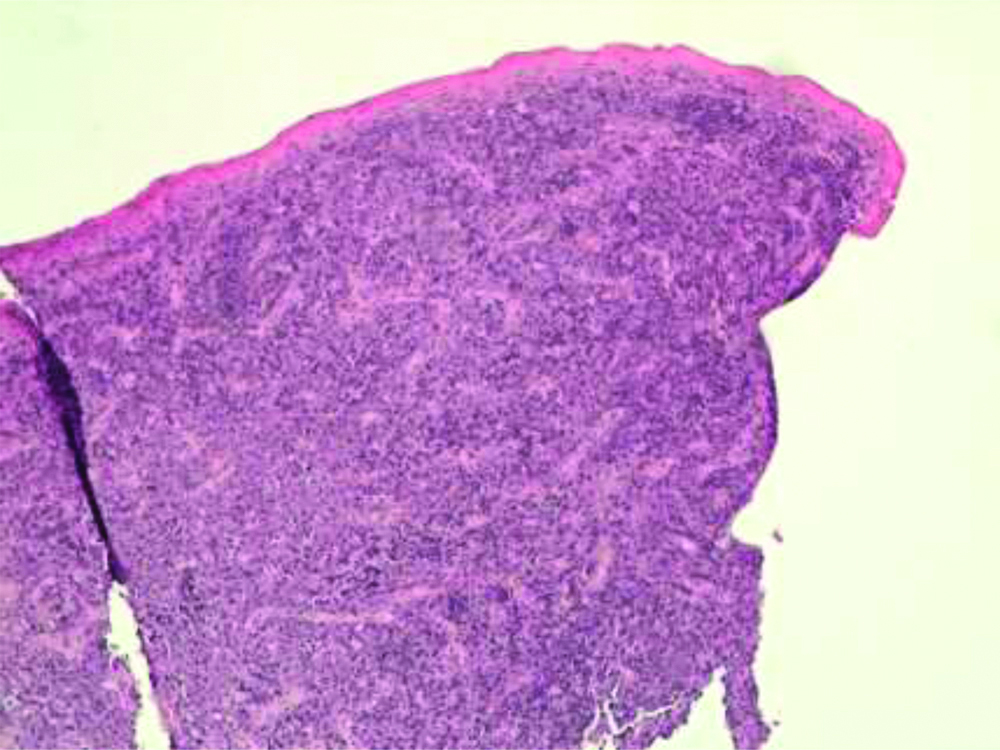
There were no monoclonal bands to urinary immunofixation, and a negative Bence Jones proteinuria. In serum immunofixation, there was a mild monoclonal component in the gamma area typified as IgG Lambda light chains in a framework of hypergammaglobulinemia. The patient had chronic esotoxic hepatopathy but with explorable superficial lymph node stations. An incisional biopsy involving the base of the lesion and the surrounding healthy gingiva was performed. The histological examination, under a Leica DMD108 microscope (Leica Camera AG, Wetzlar, Germany) found infiltration of the oral mucosa by a monomorphic population of large lymphoid elements with morphological characteristics similar to immunoblasts [Table/Fig-3].
The submucosa was infiltrated by numerous blast cells with brisk mitotic activity, and diffuse growth pattern (H&E, magnification x10).
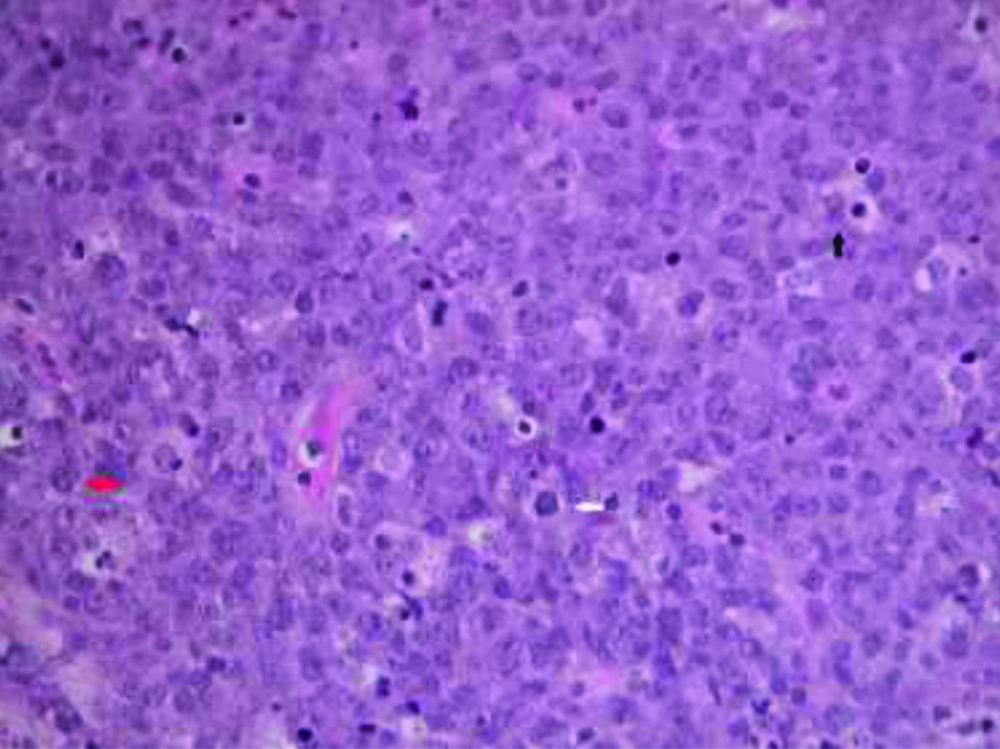
They had abundant basophilous cytoplasm, sometimes with clear paranuclear halo (“hof”), and eccentric nucleus with prominent central nucleus [Table/Fig-4]. There were cellular elements in apoptosis and macrophages with dyeable bodies that gave a “starry sky” aspect to the lesion. Atypical mitotic figures were also frequent.
The tumour cells had round and eccentric nucleus, one or more prominent nucleoli and moderate to abundant basophilous cytoplasm (see black arrow). Macrophages with dyeable bodies gave a “starry sky” aspect to the lesion (see white arrow). There were rare cells with plasmacytic differentiation, chromatin typically arranged as a wagon wheel (see red arrow) (H&E, magnification x40).
Finally, rare cellular elements with plasmacytic differentiation, with chromatin typically arranged as a wagon wheel, were found. Immunohistochemical (IHC) staining was carried out using tissues fixed in formalin and incorporated in paraffin. IHC staining showed a strong membrane immunoreactivity for CD138 [Table/Fig-5], a high related Ki-67 mitotic index of about 70% [Table/Fig-6] and a positivity for Kappa and Lambda light chains [Table/Fig-7]. Immunostaining for CKAE1/3, Synaptophysine, Chromogranin, CD56 and CD20 were negative [Table/Fig-8].
Plasma cell differentiation of the tumour cells was confirmed by diffuse and strong positivity with the plasma cell markers CD138 (magnification x10).
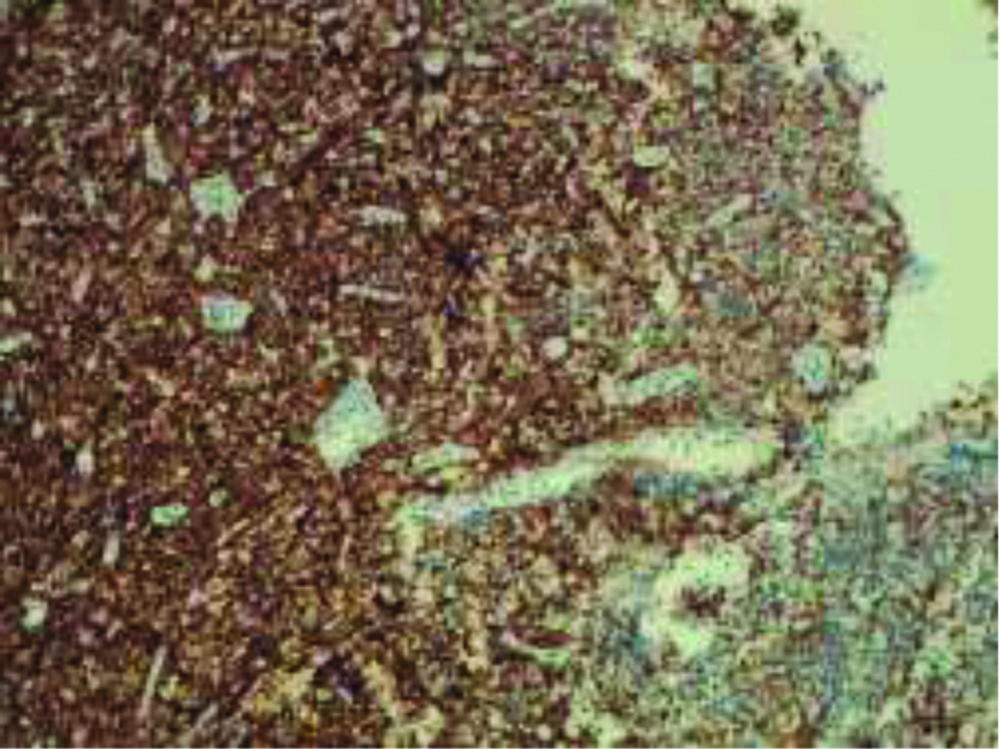
Ki-67 labeling index was approximately 70%, indicating highly proliferative activity (magnification x10).
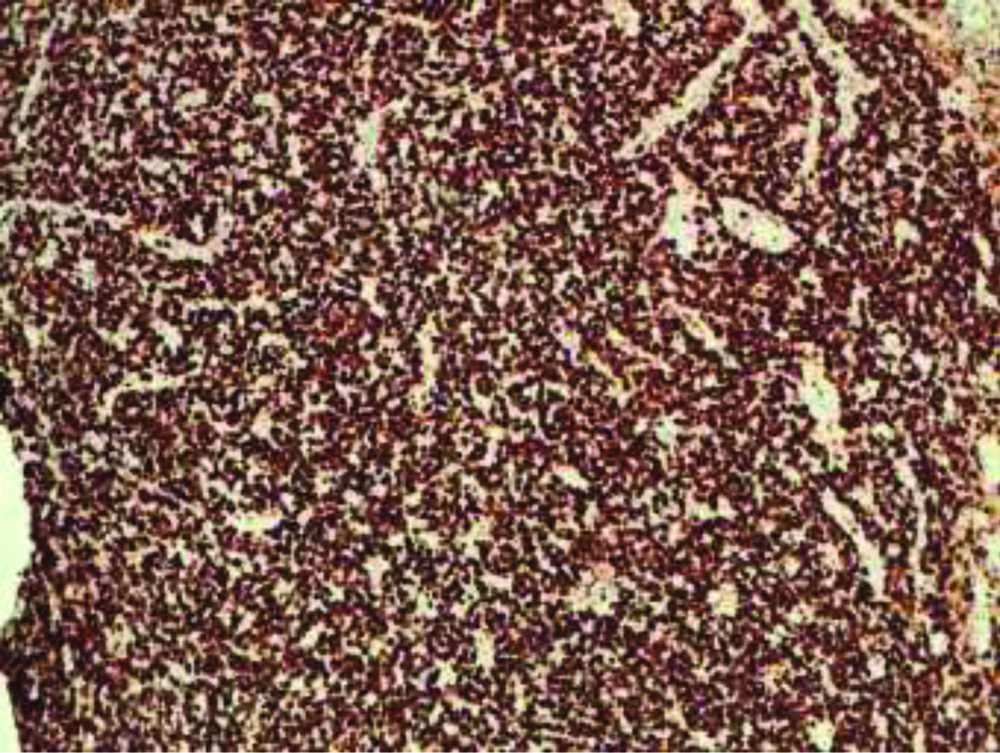
Immunohistochemical stains (IHC) with kappa (a) and lambda (b) light chains revealed kappa light chain restriction in PBL cells (magnification x20).
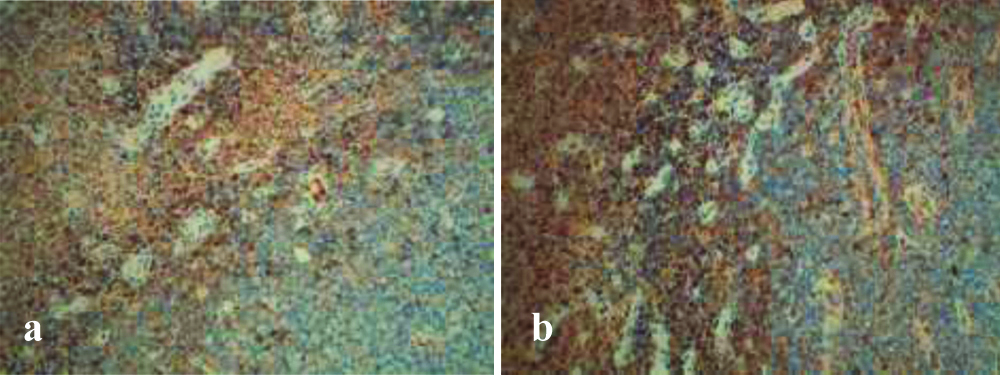
Histomorphologic features and phenotypic profile. Cells of the oral tumour were immunohistologically negative for CD20 (a, magnification x10) and CKAE1/3 (b, magnification x4), and were weakly focally positive for CD79a (c, magnification x4).
The morphological and immunophenotypic characteristics allowed the diagnosis of non-Hodgkin Lymphoma with large B cells of plasmablastic type. Lymphoma was negative to the presence of Epstein-Barr virus (EBV) RNA, researched with the hybridisation of the probe EBER-1 and negative to ‘herpes Virus Human 8 (HHV8) via PCR.
The positivity for plasmacellular markers, the high proliferative index, the absence of monoclonal plasmacellular population along with clinical features, rapid growth in the oral cavity and medullary involvement, allowed differential diagnosis of PBL from plasmacellular neoplasms.
The absence of B-cell markers oriented towards plasmablastic differentiation rather than morphologically similar immunoblastic lymphoma. The patient was referred to the Department of Haematology and underwent chemotherapy on a standard CHOP scheme (cyclophosphamide, doxorubicin, vincristine, and prednisone). At the postchemotherapy Positron Emission Tomography-Computed Tomography (PET-CT) checkup in July 2018 there was no active lymphoproliferative disease. Regression of Fluorodeoxyglucose (FDG) uptake in the left mandibular region, in the ipsilateral submandibular lymph nodes and in the lymph nodes of retropancreatic area was referred. The patient dropped-out during the follow-up.
Discussion
The PBL is a very aggressive tumour, classified by the World Health Organisation (WHO) as a variant of diffuse large B-cell lymphoma. The prognosis is severe, with an average survival, since diagnosis, of six months [1]. It is reported to have an average survival of nine months in HIV negative patients and 14 months in HIV positive patients. The increased survival of positive HIV patients could be linked to lower age, intensive chemotherapy and Chimeric Antigen Receptor T (CART) treatment [2]. Otherwise a study of 481 patients with PBL (the National Cancer Data Base) stated there was no difference in survival depending on HIV status by stratifying age [3]. It is currently unclear whether the HIV infection could play a role in the pathogenesis and progression of PBL [4].
The diagnosis of PBL can be complex due to the clinical appearance similar to other lymphoid and myeloid cancers [4]. For the diagnosis of PBL it is necessary to have a positivity for CD138, CD38 and MUM1, variable positivity for vs38c, EMA, CD79a, CD31, bcl-2, CD20, LMP1, CD43, CD45 and negativity for Igm, CD3, CD15, CD30, CD56, ALK-1, bcl-6 and EBNA-2 [5]. There is a close correlation with the state of immunosuppression caused by HIV infection or transplantation, instead only 5% of cases are immunocompetent [6].
A previous case report by Lin L et al., revealed that in 80% of HIV negative patients the localisation of PBL was extra-lymphatic, including the gastrointestinal tract, nasal and paranasal sinuses, bone, skin, lung and in 20% of the cases it was oral [7]. In the oral cavity the PBL was located on adherent gingiva in 40% of the cases or on edentulous alveolar crests, mainly in the mandible, in equal percentage between anterior and posterior region [8]. Some cases of PBL have been associated with postextractive alveolus [9-11] similar to the current case report findings. Other typical locations were observed on the hard palate, floor of the mouth and buccal mucosa with an appearance of an ulcer or an exophytic and sessile rapidly growing lobulated mass with an irregular surface [8].
A common expression of the oral PBL is asymptomatic bleeding and ulceration in stage I Ann Arbor, instead in stage IV Ann Arbor there may be paresthesia of the lower lip, hypoesthesia of the lower alveolar nerve, homolateral ear pain and trisma. Immunocompetent patients are less likely to have B symptoms, fever, night sweats, weight loss, but not specific to PBL [12]. In the early stages it could mimic inflammatory dental processes, and, in fact, in about 40% of the cases, the oral lymphomas went undiagnosed in the initial assessment. This could lead to ineffective treatments such as extractions, the use of antibiotic therapies and periodontal therapies that delay diagnosis, proper treatment and worsen the prognosis [13]. Lymph node involvement could also be confused with tonsillitis and mistakenly treated with antibiotics [14].
The average age of onset in HIV negative patients is 58 years, and a third of patients have secondary immunodeficiency due to organ transplantation. Autoimmune diseases, chronic inflammatory diseases, past neoplasms and an age of more than 60 years were identified as risk factors in immunocompetent patients, since they could promote immunosenescence. The PBL is the second most common diagnosis (24%) among oral non-Hodgkin’s lymphoma, while diffuse large B cell lymphoma is the oral main diagnosis (42%) [6]. The increase of oral PBL detection could actually be linked to the increased possibility of carrying out differential diagnosis with Anaplastic Kinase Lymphoma (ALK) large positive B-cell lymphoma, Kaposi sarcoma, Primary Effusion Lymphoma (PEL), plasmablastic Plasma Cell Myeloma (PBM), Burkitt’s lymphoma, plasmacytoma, undifferentiated carcinomas, Castleman disease-LBCL, through the identification of ALK, EBV, HHV-8. The diagnosis of PBL could be complex and often delayed. The clinical appearance and positivity to markers of immunohistochemistry are differentiating factors. Oral poorly differentiated cancer could be differentiated from PBL through its immunoreactivity to CK and EMA. Immunoblastic lymphoma, B-Cell Lymphoma (DLBCL) and Burkitt lymphoma could be differentiated from PBL through bcl-6, CD20, CD45 and PAX-5 markers [5]. The differential diagnosis between PBL and plasmablastic PBM is strongly related to the presence of concurrent clinical signs. The presence of lymphadenopathy and oropharyngeal neoformations in EBER-negative/unknown cases promotes the diagnosis of PBL [15]. The presence of serum monoclonal proteins and/or bone lithic lesions, found radiographically, suggested the diagnosis of plasmacellular myeloma compared to PBL. The presence of EBV is strongly related to PBL compared to plasmacellular tumours [16]. The prognosis is poor compared to other lymphomas with an average survival time of 6-19 months, probably linked to over-expression of the oncogene MYC and decreased expression of the gene p53. The mutation of the gene PRDM1, could contribute to the over expression of MYC, resetting the expression of the protein Blimp 1 [17].
Patients with oral PBL have been treated heterogeneously and there is no standardised and unanimally accepted medical treatment. Treatment may consist of chemotherapy for the disseminated disease and radiotherapy in oral localised non-Hodgkin lymphoma [18]. Common immunotherapeutic agents includes Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP), Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin, Dexamethasone, and High-Dose Methotrexate and Cytarabibe (Hyper-CVAD-MA), Cyclophosphamide, Vincristine, Doxorubicin, High-Dose Methotrexate/Ifosfamide, Etoposide, and High-Dose Cytarabine (CODOX-M/IVAC), and infusional Etoposide, Prednisone, Vincristine, Cyclophosphamide, and Doxorubicin (EPOCH) [19]. Potential therapeutic approaches for patients with PBL may include Bortezomib and Lenalidomide (widely used for the treatment of multiple myeloma), Siltuximab, Tozilizumab, Brentuximab vedotin and autologous stem cell transplantation [20]. The selection of therapy is considered on a case-by-case basis depending on the stage of the oral lymphoma, the presence of systemic symptoms or the association with HIV infection.
Conclusion(s)
The PBL is a very aggressive B lymphocyte tumour with a severe prognosis, relatively common in HIV positive patients. It shows an average survival period, since diagnosis, of about 14 months, even shorter, inexplicably, in HIV negative patients. The clinical features of this neoformation are poorly pathognomonic and therefore the diagnosis remains complex and often unexpected. While the presence of immunodeficiency or autoimmune inflammatory diseases may suggest the inclusion of this form among others, the diagnosis of oral PBL in immunocompetent patients, with no concomitant pathologies, remains a challenge. Future investigations, based on clinical and immunophenotypic features, should be designed to help elucidate the specific pathogenetic mechanisms of oral PBL in immunocompetent patients, aiming to identify the molecular targets for the treatment of this rare lymphoid disease.
[1]. Loghavi S, Alayed K, Aladily TN, Zuo Z, Ng SB, Tang G, Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: A clinicopathologic analysis of 61 patientsJ Hematol Oncol 2015 8:6510.1186/s13045-015-0163-z26055271 [Google Scholar] [CrossRef] [PubMed]
[2]. Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphomaLeuk Lymphoma 2010 51(11):2047-53.10.3109/10428194.2010.51604020919850 [Google Scholar] [CrossRef] [PubMed]
[3]. Qunaj L, Castillo JJ, Olszewski AJ, Survival of patients with CD20-negative variants of large B-cell lymphoma: An analysis of the National Cancer Data BaseLeuk Lymphoma 2018 59(6):1375-83.10.1080/10428194.2017.138791229019447 [Google Scholar] [CrossRef] [PubMed]
[4]. Kessler AJ, Marcellino BK, Niglio SA, Petersen BE, Malone AK, A rare presentation of HIV-negative plasmablastic lymphoma: A diagnostic dilemmaCase Rep Hematol 2019 2019:290731710.1155/2019/290731730906602 [Google Scholar] [CrossRef] [PubMed]
[5]. Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, Maestre L, Sanchez-Verde L, Roncador G, Aggressive large B-cell lymphoma with plasma cell differentiation: Immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotypeHaematologica 2010 95(8):1342-49.10.3324/haematol.2009.01611320418245 [Google Scholar] [CrossRef] [PubMed]
[6]. Tchernonog E, Faurie P, Coppo P, Monjanel H, Bonnet A, Algarte Génin M, Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: Analysis of 135 patients from the LYSA groupAnn Oncol 2017 28(4):843-48.10.1093/annonc/mdw68428031174 [Google Scholar] [CrossRef] [PubMed]
[7]. Lin L, Zhang X, Dong M, Li L, Wang X, Zhang L, Human immunodeficiency virus-negative plasmablastic lymphoma: A case report and literature reviewMedicine (Baltimore) 2017 96(7):e617110.1097/MD.000000000000617128207555 [Google Scholar] [CrossRef] [PubMed]
[8]. Bhattacharyya S, Bains APS, Sykes DL, Iverson BR, Sibgatullah R, Kuklani RM, Lymphoid neoplasms of the oral cavity with plasmablastic morphology-A case series and review of the literatureOral Surg Oral Med Oral Pathol Oral Radiol 2019 128(6):651-59.10.1016/j.oooo.2019.08.00131494113 [Google Scholar] [CrossRef] [PubMed]
[9]. Deshmukh R, Abhyankar P, Mhapuskar A, Varpe H, Intraoral plasmablastic lymphoma as a primary oral manifestation: A case report and review of literatureJ Oral Maxillofac Pathol 2020 24(Suppl 1):S91-96.10.4103/jomfp.JOMFP_294_1932189913 [Google Scholar] [CrossRef] [PubMed]
[10]. Tzankov A, Brunhuber T, Gschwendtner A, Brunner A, Incidental oral plasmablastic lymphoma with aberrant expression of CD4 in an elderly HIV-negative patient: How a gingival polyp can cause confusionHistopathology 2005 46(3):348-50.10.1111/j.1365-2559.2004.01990.x15720425 [Google Scholar] [CrossRef] [PubMed]
[11]. Coskunses FM, Cilasun Ü, Topcu PC, Tokuc B, Primary diffuse large B-cell lymphoma of the mandible: A case reportGerodontology 2020 37(3):307-11.10.1111/ger.1247032809252 [Google Scholar] [CrossRef] [PubMed]
[12]. Liu M, Liu B, Liu B, Wang Q, Ding L, Xia C, Human immunodeficiency virus-negative plasmablastic lymphoma: A comprehensive analysis of 114 casesOncol Rep 2015 33(4):1615-20.10.3892/or.2015.380825695332 [Google Scholar] [CrossRef] [PubMed]
[13]. Hassona Y, Almuhaisen G, Almansour A, Scully C, Lymphoma presenting as a toothache: A wolf in sheep’s clothingBMJ Case Rep 2017 2017:bcr201621868610.1136/bcr-2016-21868628119440 [Google Scholar] [CrossRef] [PubMed]
[14]. Richards A, Costelloe MA, Eveson JW, Scully C, Irvine GH, Rooney N, Oral mucosal non-Hodgkin’s lymphoma-a dangerous mimicOral Oncol 2000 36(6):556-58.10.1016/S1368-8375(00)00047-6 [Google Scholar] [CrossRef]
[15]. Ahn JS, Okal R, Vos JA, Smolkin M, Kanate AS, Rosado FG, Plasmablastic lymphoma versus plasmablastic myeloma: An ongoing diagnostic dilemmaJ Clin Pathol 2017 70(9):775-80.10.1136/jclinpath-2016-20429428249941 [Google Scholar] [CrossRef] [PubMed]
[16]. Castillo JJ, Bibas M, Miranda RN, The biology and treatment of plasmablastic lymphomaBlood 2015 125(15):2323-30.10.1182/blood-2014-10-56747925636338 [Google Scholar] [CrossRef] [PubMed]
[17]. Montes-Moreno S, Martinez-Magunacelaya N, Zecchini-Barrese T, Villambrosía SG de, Linares E, Ranchal T, Plasmablastic lymphoma phenotype is determined by genetic alterations in MYC and PRDM1Mod Pathol 2017 30(1):85-94.10.1038/modpathol.2016.16227687004 [Google Scholar] [CrossRef] [PubMed]
[18]. Pinnix CC, Shah JJ, Chuang H, Costelloe CM, Medeiros LJ, Wogan CF, Doxorubicin-based chemotherapy and radiation therapy produces favorable outcomes in limited-stage plasmablastic lymphoma: A single-institution reviewClin Lymphoma Myeloma Leuk 2016 16(3):122-28.10.1016/j.clml.2015.12.00826795083 [Google Scholar] [CrossRef] [PubMed]
[19]. Katchi T, Liu D, Diagnosis and treatment of CD20 negative B cell lymphomasBiomark Res [Internet] 2017 Feb 7 [cited 2021 Jan 12] :5Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5297138/10.1186/s40364-017-0088-528191314 [Google Scholar] [CrossRef] [PubMed]
[20]. Lopez A, Abrisqueta P, Plasmablastic lymphoma: Current perspectivesBlood Lymphat Cancer 2018 8:63-70.10.2147/BLCTT.S14281431360094 [Google Scholar] [CrossRef] [PubMed]