Dengue is endemic in all continents in recent years and has, rapidly emerged and the most important mosquito-borne viral disease. The dengue viruses are arbovirus; categorised under genus Flavivirus. DENV genome is positive-sense, single stranded Ribonucleic Acid (RNA), codes for two types of viral proteins: (I) Structural proteins: Capsid (C) protein, Membrane (M) protein, Envelop (E) protein, (Π) Nonstructural proteins like NS1, NS2a, NS2b, NS3, NS4a, NS4b and NS5 [1-3]. Dengue virus has four serotypes as DENV-1 to DENV-4. Recently, 5th serotype was identified from Bangkok in 2013. All these serotypes are circulated either singly, or more than one; at the same time, so persons living in a dengue endemic area can have all four serotype infections during their lifetimes. DENV infection causes Dengue Fever (DF) with or without warning sign. A severe dengue includes Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) in which thrombocytopenia, haemorrhage and excessive plasma leakage occurs which leads to death [4,5].
Dengue virus are antigenically distinct and extensive cross-reactivity was seen in serological assays due to all flavivirus has common group epitopes on envelop protein. One DENV serotype infection provides lifelong immunity to that serotype but does not protect against infection from another DENV serotype [6]. In DENV infection, the antibody dependent enhancement phenomena production leads to increase in severity of secondary dengue infection [7]. The first epidemic dengue was recorded in 1780 at Chennai. The first epidemic of DF proved virologically in 1963-1964 at Calcutta and Eastern Coast area [8-10]. Generally, one dominating serotype causes outbreak, but circulation of all four serotypes are also found, that leads to mixed infection with more than one serotype and increased disease severity [11-14]. Co- infection of all four DENV serotypes with each other in single outbreak were reported in 2017, from Odisha and Hyderabad [15,16].
In recent year various method for diagnosis of DENV infection was developed like virus isolation, Molecular method (nucleic acid amplification tests; e.g., RT-PCR, Real-time RT-PCR), and serological method e.g., Antibody detection (by Haemagglutination Inhibition (HAI), Complement Fixation Test (CFT) and ELISA for IgM or IgG detection), Antigen detection (by ELISA and Immunochromatographic Test (ICT) or direct-Immunofluorescence (IF)) [17]. For DENV serotyping antigen detection immunofluorescence assay and plaque reduction neutralisation were available [18], but they need well-equipped laboratories so, not widely available. Molecular methods need high specificity, sensitivity and rapid, also be serotype specific so, World Health Organisation (WHO) recommended for the detection of DENV RNA [19]. Molecular methods are becoming more widely used, it provides results more quickly and more sensitive for serotyping, all four dengue serotypes detected by fluorogenic probe-based PCR assay [7].
Knowledge about geographic distribution of disease, their burden, prevalence and incidence are necessary, and also with this background, circulating DENV serotypes strains were detected. So, these information help in appropriate utilisation of existing and emerging prevention control strategies and introduction of dengue vaccine in near future. The present study was done with an aim to identify prevalent dengue serotype and co-circulation of all the four serotype of dengue virus in endemic region of Saurashtra, Gujarat, India.
Materials and Methods
The present study was a cross-sectional study conducted after obtaining permission from the Institutional Ethical Committee (MP Shah Govt. Medical College and Guru Gobind Singh Government Hospital, Jamnagar; IEC/Certi/138/08/2018). Cases were mainly referred from Outpatient Department (OPD) and indoor of GG hospital, Jamnagar, Gujarat. India.
Sample size calculation: A pilot study for clinically, suspected dengue cases conducted for period of one month gave estimated proportion, of 7.6% in Saurashtra region. Considering 7.6% of proportion sample size was calculated by formula, with vonfidence interval=95% and power of study=80%
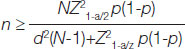
n=12086 (where, P is estimated proportion=7.6%; Z=1.96
N is population size of Saurashtra=32,56,800 as per Census 2011 [20].
d is Marginal error rate taken as 10% of estimated proportion=0.76%
Alph (@) is type I error=5% power of study
Inclusion criteria: All dengue virus antigen- NS1 and antibody-IgM ELISA positive samples were included in this study.
Exclusion criteria: ELISA negative (antigen- NS1 and antibody- IgM) were excluded for serotyping.
Study Procedure
Universal sampling method was adopted, where total 12,563 clinically suspected dengue samples were collected for period of two year (from January 2019 to December 2020) and analysed later after completion of collection of data. Samples were collected at central laboratory of GG Hospital and serotyping done at Microbiology Department of MP Shah Government Medical College, Jamnagar. After receiving blood samples, non-haemolysed, non-turbid and non-lipemic serum was selected and proceed for Dengue NS1 Ag by Microlisa test kit (if H/O of illness <5 days) and Dengue IgM Ab by NIV Pune test kit (if H/O of illness >5 days). After serological confirmation, 151 ELISA positive samples from different geographical area were selected for Dengue specific RT-PCR serotyping.
Protocol for Dengue Specific RT-PCR is as Follows
Viral RNA extraction: RNA isolation was performed using QI Amp Viral RNA mini kit (Qiagen, Valencia, CA, USA).
Pipet 560 μL prepared Buffer AVL in to microcentrifuge tube, add 140 μL serum and mix by vertexing for 15 seconds, incubate at room temperature (15-25°C) for 10 minutes, centrifuge the tube to remove drops from the inside of the lid. This was followed by a brief centrifugation; RNA precipitation was done using 560 μL of ethanol (96%-100%) to the sample and mixing by pulse-vertexing for 15 seconds and the mixture was transferred to QIAamp ® Mini column for RNA isolation. The final RNA was eluted in 60 μL of the elution buffer.
Master mix preparation for dengue differentiation: Calculate the amount of each reagent to be added for Master mix. Use 12.5 μL buffer, 1.5 μL Primer-probe Mix and 1 μL Enzyme.
Preparation of a 96 Well Plate
Take a 96 well plate, add 15 μL of the master mix in the wells, add 10 μL of the extracted RNA, negative control and the positive control. Mix properly, cover the well with adhesive film, slightly vortex the plate and centrifuge briefly afterward and put the strip in ABI-7500 RT-PCR.
Detector Programming
For pathogen DEN-1 FAM dye at 520 nm, DEN-2 VIC dye at 550 nm, DEN-3 ROX dye at 610 nm and DEN-4 CY5 dye at 670 nm wavelength used.
Temperature Cycle
Reverse Transcription at 15 minutes at 50°C;
Taq mix inhibitor inactivation at one minute at 94°C; and
PCR amplification (for 45 Cycles) at 94°C for 8 Seconds 60°C for one minute* (data collection).
Interpretation
Negative controls: All primers and probes should not exhibit amplification curves that cross the threshold line.
Positive controls: Must show amplification with sigmoid curve for specific primer and probes sets: DEN-1, DEN-2, DEN-3 and DEN-4.
Internal/Extraction controls: Must show amplification to specific primer and probes.
RNase P (RP): All clinical specimen shows amplification curves in RP reaction and cross the threshold line, indicating the presence of the human RNase P gene. (If all primer and probes shows amplification but not RNase P result valid, but all marker and RP not amplified result as an inconclusive). If the criteria listed above are met, any patient sample displaying an exponential trace should be considered as positive for one of the pathogens targeted by the kit.
Statistical Analysis
Percentage, Chi-square test (Yates correction applied where cell value below 5). Significance taken at p<0.05; data were entered and analysed in Microsoft excel 2016.
Results
Total 12,563 clinically suspected dengue samples tested, 4069 (32.3%) had confirmed Dengue positive by ELISA method. Of these, 151 dengue positive samples tested for RT-PCR serotyping and only 109 were dengue positive, negative result does not preclude infection because patient have low viral load, and that was beyond detectable limit of the assay.
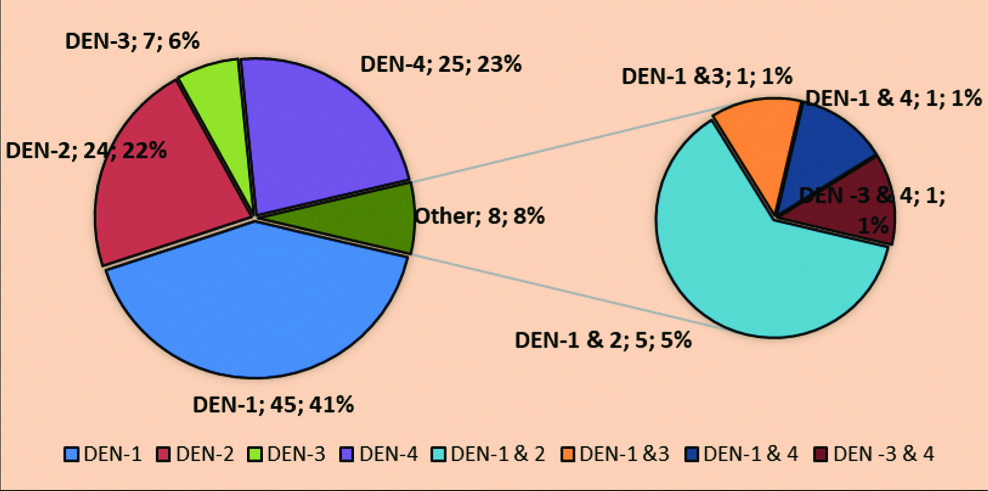
In dengue serotyping study all the four serotypes in circulation. DENV-1 was 41.2%, followed by DENV-4 was 23%, DENV-2 was 22% and DENV-3 was 6.4%. Also, mixed infection of DENV-1 and DENV-2, DENV-1 and DENV-3, DENV-1 and DENV-4 and DENV-3 and DENV-4 were 8% [Table/Fig-1].
Serotype distribution of dengue cases in Saurashtra region.
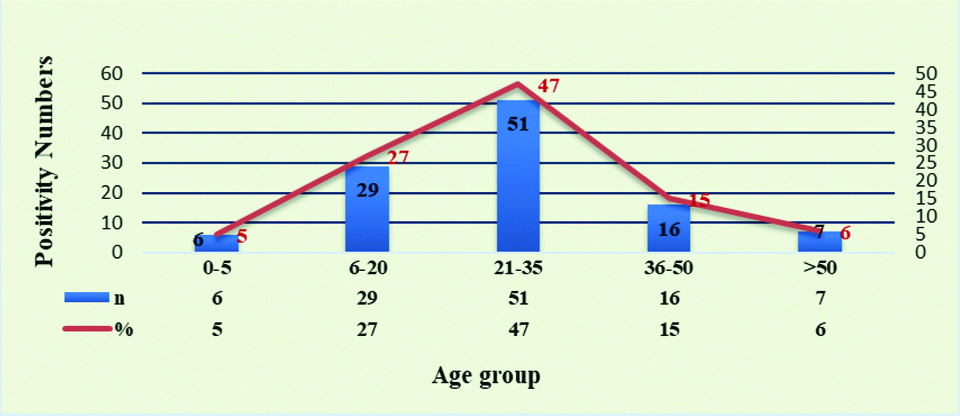
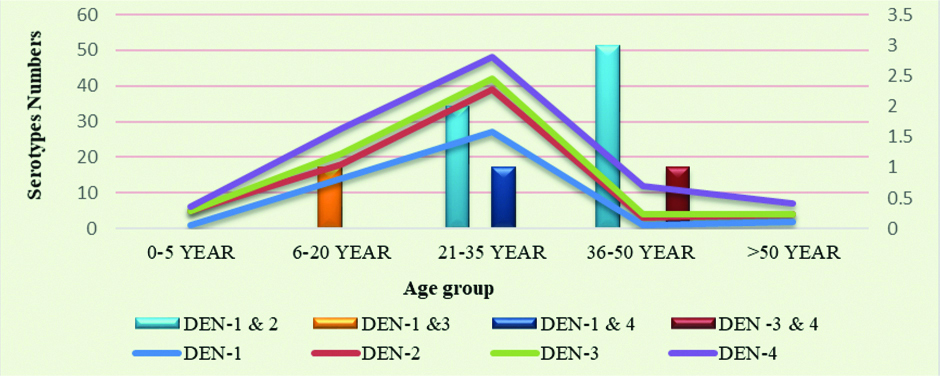
In male/female suspected dengue cases positivity percentage in male 65 (60%) was higher than female 44 (40%), This difference between male and female among specific DENV-1 to DENV-4 was statistically not significant (p>0.05) [Table/Fig-2]. Majority of samples were in age group 21-35 year (47%) followed by 6-20 year (27%). Most of co-infection were in age group 21-35 year and 36-50 year [Table/Fig-3,4].
Gender wise distribution of dengue positive cases serotyping.
| Serotype | Male (%) | Female (%) |
|---|
| DENV-1 | 28 (25.7) | 17 (13.8) |
| DENV-2 | 12 (11) | 12 (11) |
| DENV-3 | 3 (2.7) | 4 (3.6) |
| DENV-4 | 16 (14.7) | 9 (8.2) |
| DENV-1 and DENV-2 | 3 (2.7) | 2 (1.8) |
| DENV-1 and DENV-3 | 1 (1) | 0 |
| DENV-1 and DENV-4 | 1 (1) | 0 |
| DENV-3 and DENV-4 | 1 (1) | 0 |
| Total | 65 (60) | 44 (40) |
Chi-square (c2)=1.97, d.f=3, p.0.05, statistically not significant. Chi-square calculated only for specific DENV-1 to DENV-4 infection and not for mixed infection
Age wise positivity number (%) of dengue cases.
Chi-square value=1.82, p-value=0.77, statistically not significant
Age wise serotyping of dengue cases.
Chi-square value =16.74, p=0.01, statistically significant. Calculation done between groups, 0-20 years, 21-35 years and >35 years: Chi-square calculated only for specific DENV-1 TO DENV-4 infection and not for mixed infection
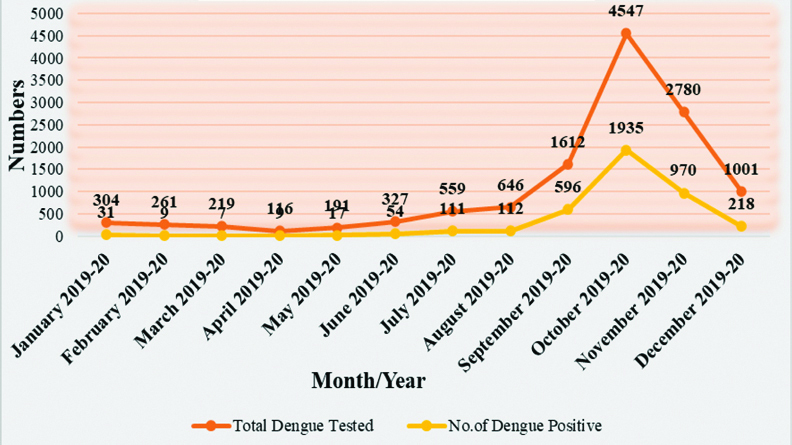
Sporadic cases were observed throughout of year, but cases were gradually increasing with positivity from June and reach to peak in October (47%) than gradually declining of cases in November [Table/Fig-5].
Month wise distribution of dengue cases in Saurashtra region.
Chi-square value=493.43, p=0.0001, statistically significant. Chi-square calculated for quarters Jan-Mar 2019-2020, Apr-June 2019-2020, Jul-Sept 2019-2020, Oct-Dec 2019-2020.
Majority showed dengue positive cases in three to four days duration from the onset of illness and co-infection with more than one serotype was after three days duration of illness noted [Table/Fig-6].
Positivity in duration (in days) of onset of illness.
| Distribution of dengue serotypes as per duration (in days) from the onset of illness |
|---|
| Serotypes | 1 | 2 | 3 | 4 | 5 | >5 |
|---|
| DEN-1 | 0 | 9 | 10 | 21 | 5 | 0 |
| DEN-2 | 0 | 1 | 9 | 9 | 4 | 1 |
| DEN-3 | 2 | 0 | 2 | 2 | 1 | 0 |
| DEN-4 | 1 | 5 | 8 | 7 | 4 | 0 |
| DEN-1 and 2 | 0 | 0 | 2 | 3 | 0 | 0 |
| DEN-1 and 3 | 0 | 0 | 0 | 1 | 0 | 0 |
| DEN-1 and 4 | 0 | 1 | 0 | 0 | 0 | 0 |
| DEN -3 and 4 | 0 | 0 | 1 | 0 | 0 | 0 |
Discussion
Genus flavivirus have highest mutation rate tends to have chances of co-infection much higher because all four dengue serotypes circulate in a population. Study shows increasing trend of co-circulation of different DENV serotypes in region that suggest Saurashtra becoming hyper endemic region; so, monitoring of circulatory strain is required. Since, DENV-2 and DENV-3 were causing severe diseases than the other [4] so, rapid and accurate detection of serotype can help in DENV infection detection. For this DENV RNA detection and with the real-time RT-PCR serotyping during the first five to six days after symptomatic onset. So, timely dengue virus infection detection, their serotype status help to initiate timely community and vector control programs to mitigate the spread of DENV infection.
Present study, shows the highest monotypic infection was DENV-1 (41%), followed by DENV-4 (23%), DEN-2 (22%) and DENV-3 (6%) provides direct evidence of all four serotypes were circulated in Saurashtra region, similar finding were observed in Vicente CR et al., shows DENV-1 (77.3%), Rahman M et al., (72.7%) and Paul R et al., shows (57%), but not corelated with Racherla RG et al., and Sarkar A et al., study shows highest monotypic infection was DENV-2 (41%), and (60%), and Mehta TK and Shah PD, study shows DENV-3 (53%) [21-26].
In present study, DEN-4 (23%) were reported after DEN-1 monotypic infection, which was similar to Vicente CR et al., showed 16.1%, Racherla RG et al., showed 37% [21,24]; While Mehta TK and Shah PD, showed 3% [26], Paul R et al., and Sarkar A et al., study were no any GEN-4 serotype found [23,25]. All these finding shows that in different region different serotypes were circulated can shift from one to another, but in present study samples were obtained from hospitalised patients. So, result may not actually reflect the predominant serotype.
In this study, few cases DENV-3 (6%) infection were detected similar to Racherla RG et al., DENV-3 (12%) [24] and Vicente CR et al., DENV-3 (0.2%) [21] while in Sarkar A et al., [25], Paul R et al., and Mehta TK and Shah PD, shows higher infection of DEN-3 (29.7%), (40%) and (53%) [23,26]. Prevalence rate of all four serotypes were changed in different area. So, data on serotypes help in surveillance systems in that particular area to predict an impending dengue outbreak. DF were complex epidemiology so, prevalent strain substantially changed it may be due to urbanisation, population growth, lack of sanitisation, ineffective mosquito controls all these facilities fertile breeding ground to vector mosquitoes.
Mixed infection was also detected in present study of different serotype was 8 (7%) of this DENV-1 and DEN-2 (5%) was predominant than another mixed infection. Mehta TK and Shah PD, [26] showed co-infection DEN-2 and DEN-3 (7.4%) and DEN-3 and DEN-4, DEN-2 and DEN-4 was (0.7%), another study of Kerala [11] reported a DEN-1 and DEN-3 co-infection was predominant than the other combinations, Rahman M et al., also showed mixed infection of DEN-1 and DEN-2 was (15.1%) [22] and Sarkar A et al., shows DEN-2 and DEN-3 (30.6%) [25]. In India, dengue is hyperendemic and all DENV serotype circulate during the same period of time that tends to mixed infection was occurred with different serotypes in the patients [27].
Seropositivity in male (60%) was higher than female in present study which was similar to the studies from Rahman M et al., showed 73.6% [22], Mehta TK and Shah PD, (61.48%) [26] and Paul R et al., (64%) [23], in opposite Sarkar A et al., showed female (46.5%) were more affected than male [25] and Racherla RG et al., were no significant association found between male and females [24]. Among all the age group middle age group (47%) was most affected in showed study was comparable to most of studies like Rahman M et al., showed 73.6% [22], Mehta TK and Shah PD, showed 40.7% [26], Racherla RG et al., showed 52.7% [24] and Paul R et al., showed 42% [23]. This age groups highly comprises to working and student population and they were exposed to external environment and Ades mosquito vectors which are day biters.
Majority of dengue positivity was seen 2-5 days duration from the onset of illness in this study, which was similar to Mehta TK and Shah PD [26]. Stage of diseases important like initial phase, first 5 days of illness high number of circulating viruses are present in serum to explore the prognostic markers of severe diseases and early detection of different dengue serotype.
In present study, dengue virus positivity increases from monsoon and at peak level in post-monsoon period were similar to various studies [22,26]. This can be explained by mosquito vector multiply during monsoon period and stagnant of water sources in post monsoon period leads to increases favoring of breeding mosquito vectors. So, appropriate and effective preventive measures should be taken during these time period.
Limitation(s)
The sample was collected mostly from a single center, so serotype prevalent in present study may vary from the other region. The clinical requisition form of patients were received in our the center was not completely filled by the clinician so, clinical manifestation and severity of diseases could not be assessed.
Conclusion(s)
All four dengue virus serotypes can co-circulate in region and the immunity to one serotype does not give protection to another heterotopous serotype. Seasonal peak which coincides with increased vector breeding’s during monsoon and post-monsoon period. So, effective implementation of vector control measures and personal prophylaxis is effective to control diseases. This should be concerns to public health authority and should stimulate the arbovirus diagnostic method in public health laboratories. People should use mosquito repellents and nets to avoid being infected by mosquitoes spreading dengue, malaria, chikungunya and other diseases. Prevention is always better than cure, help themselves by keeping the surroundings clean and not letting garbage accumulate.
Chi-square (c2)=1.97, d.f=3, p.0.05, statistically not significant. Chi-square calculated only for specific DENV-1 to DENV-4 infection and not for mixed infection