Introduction
The ovaries are the primary female reproductive organs and endocrine glands. Ovarian carcinoma has often been called as a silent killer because the symptoms may develop so late that the chances of cure are very poor. According to World Health Organisation (WHO) ovarian tumours are classified based on their most probable tissue of origin: surface epithelial (65%), germ cell (15%), sex cord-stromal (10%), metastases (5%) and miscellaneous. The malignant surface epithelial tumours are further classified by cell type into serous, mucinous, endometrioid, clear cell, brenner, seromucinous and undifferentiated carcinoma. The most widely used tumour marker in ovarian carcinoma is CA-125 which is considered as gold standard.
Aim : To find the utility of serum CA-125 levels in histopathological variants of malignant surface epithelial tumours, degree of differentiation and their distribution according to clinical data pertaining to age, parity, history of use of oral contraceptive pills/ovulation inducing drugs and family history of carcinoma ovary/breast or colon.
Materials and Methods
A prospective study (cohort study) was done at Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India over a period of 1.5 year (April 2017-October 2018) on 50 ovarian masses which were diagnosed as ovarian carcinoma. Data was represented as frequencies and percentages for categorical variables and as means and standard deviations for continuous variables. Analysis was done using Statistical Package for Social Sciences (SPSS) v 20.0.0.
Results
Serous carcinoma (80%) topped among all the histological variants. Serous high grade carcinoma was more common than serous low grade carcinoma. Maximum rise of serum CA-125 levels were seen in serous carcinoma. Among serous carcinomas, mean serum CA-125 levels were more in high grade serous carcinoma than low grade serous carcinoma and the results were statistically significant.
Conclusion
Serum CA-125 level is a great tool for diagnosis, follow-up and prognosis of ovarian carcinomas.
Introduction
Ovaries are the primary female reproductive organs and endocrine glands [1]. It is complex in its embryology, histology, steroidogenesis and has potential to develop malignancy [2]. The ovarian tumours are common form of neoplasia in women and account for 30% of female genital cancers [3]. The incidence of ovarian carcinoma ranks third, below only to the carcinoma of cervix and endometrium [2]. Asian countries have an incidence of 2-6 new cases per 1,00,000 women per year [4]. In India, the ovarian carcinoma incidence (age-adjusted rate per 1,00,000) in different population-based cancer registries is reported to range from 1.7 to 15.2 for the year 2012 to 2014. An increasing trend of this carcinoma has been observed since 1982 till date. The projected number of cases for this carcinoma in India for 2015 and 2020 are 45,231 and 59,276, respectively [5]. Among all the ovarian tumours, 75% are benign and 25% are malignant [6].
Null parity increases the risk of ovarian carcinoma while oral contraceptive use, pregnancy and lactation have got a protective role [7].
A strong family history of ovarian or breast carcinoma along with colon carcinoma has a strong predisposition to the development of ovarian carcinoma. The two most common genes involved in this carcinoma are BRCA1 and BRCA2. Ovarian carcinomas associated with germline mutations appear to be predominantly of serous type and age of the patient at the time of diagnosis is significantly less as compared to the sporadic ovarian carcinomas [8].
Ovarian carcinoma has often been called as the silent killer because the symptoms may develop so late that the chances of cure are very poor [9].
The World Health Organisation (WHO) histological classification for ovarian tumours separates ovarian neoplasms according to the most probable tissue of origin: surface epithelial (65%), germ cell (15%), sex cord-stromal (10%), metastases (5%) and miscellaneous [10]. The malignant surface epithelial tumours are further classified by cell type into serous, mucinous, endometrioid, clear cell, brenner, seromucinous and undifferentiated carcinoma [11]. Initial diagnosis of carcinoma ovary is basically based on three things. These are ultrasound guided Fine Needle Aspiration Cytology (FNAC) proven cases, raised CA-125 and those having malignant effusion [12]. Beside these initial diagnostic criteria the definitive diagnosis is based on the histopathological examination.
The most widely used tumour marker in ovarian carcinoma is CA-125 which is considered as gold standard [13]. The discovery of OC125, an antibody that recognises CA-125, was made by Bast, Knapp and colleagues in 1981 [14]. CA-125 levels of less than 35U/ml are accepted as normal [13].
The pretreatment value of CA-125 must be at least twice the upper limit of the reference range. Test should be done two weeks prior to treatment. Further samples are taken at 2nd and 4th week after the treatment and at 2-3 weeks intervals thereafter [15].
It is used in cases of surface epithelial malignancy and more important in serous type of ovarian tumour than mucinous. It is useful in follow-up cases also. The CA-125 levels are elevated in 85% of serous, 65% of endometrioid, 40% of clear cell and 36% of undifferentiated carcinomas, but it is elevated only in 12% of ovarian mucinous carcinomas [16].
The early detection of epithelial ovarian carcinoma is essential for prolonging the survival. Epithelial malignancies can possibly be detected at an early stage by means of blood testing, because as many as 50% of Stage I and 90% of patients with advanced ovarian carcinoma are associated with elevated blood levels of CA-125 [14].
The present study was undertaken to find the role of serum CA-125 levels in histopathological variants of ovarian carcinoma, their degree of differentiation and to associate histopathological types (WHO classification) of ovarian carcinoma with clinical data pertaining to age, parity, history of use of oral contraceptive/ovulation inducing drugs and family history of carcinoma ovary/ breast or colon.
Materials and Methods
A prospective study (cohort study) was done at Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India over a period of one and half years (April 2017-October 2018) on 50 cases of ovarian masses which were histopathologically diagnosed as ovarian carcinoma. In this study a non probability sampling was applied and as per feasibility 50 patients were considered. Ethical approval was taken i.e., (BFUHS/2K17p-TH/216). Consent was taken from all the patients.
Inclusion criteria: Patients presenting with any of the following: All patients with adnexal mass on radio imaging findings, FNAC proven ovarian carcinoma, positive fluid cytology suggestive of ovarian carcinoma and elevated CA-125 levels: twice the normal values.
Exclusion criteria: Imaging findings suggestive of benign lesion or all conditions associated with raised CA-125 levels except ovarian carcinoma.
Study Procedure
Relevant clinical history (age, parity, history of use of oral contraceptive pills/ovulation inducing drugs and family history of carcinoma ovary/breast or colon) was recorded from histopathological proforma as well as from the patients record. The ovarian specimens received were subjected to tissue processing as per the standard protocol. The specimens were fixed in 10% neutral buffered formalin and processed by fully automatic tissue processor. Routine processing was followed by block making, paraffin tissue sections cutting, DPX mounting and staining by Haematoxylin and Eosin (H&E) method. Slides were prepared and examined under light microscope.
Measurement of CA-125
Specimen Collection and Preparation: 2 mL venous blood was drawn from each subject under aseptic conditions in plain vial for CA-125 level investigation. After the formation of blood clot, centrifugation was done at 3000 rpm for ten minutes to separate serum. Within two hours after centrifugation, at least 500 μL of cell-free sample was transfered to a storage tube. A tight stopper was put on the tube immediately. Samples were stored with tight stoppers at room temperature (15 to 30°C) for no longer than eight hours.
CA-125 level measurement was done on fully automated analyser Beckmann coulter Access OV Monitor. Access OV Monitor assay is a paramagnetic particle, chemiluminescent immunoassay for the quantitative determination of CA-125 antigen levels in human serum and plasma using the Access Immunoassay Systems.
Principle
The Access OV Monitor assay is a two-site immunoenzymatic (“sandwich”) assay. A sample is added to a reaction vessel along with mouse monoclonal anti-CA-125 antigen alkaline phosphatase conjugate and paramagnetic particles coated with a second mouse monoclonal anti CA-125 antigen antibody. The CA-125 antigen in the sample binds to the immobilised monoclonal anti CA-125 antigen on the solid phase, while the conjugate antibody reacts with a different antigenic site on the CA-125 antigen molecule [17].
Statistical Analysis
The data pertaining to clinical details was entered in the form of data matrix in Microsoft Excel and analysed using SPSS v 20.0.0. The descriptive statistics for categorical variables was represented in the form of frequencies and percentages and as means and standard deviations for continuous variables. The association between categorical variables was explored using Pearson’s Chi-square test or Fisher-Exact test wherever appropriate. The difference of continuous variables across two groups was analysed using independent samples test or Mann Whitney U test depending upon the data meeting the assumptions for the application of these tests. A p-value of <0.05 was considered as statistically significant for the purpose of this study.
Results
In the present study, majority of the cases (50%) were in age group 41-50 years with mean age of 51.82 years. Mean age for serous, mucinous, endometrioid, clear cell carcinoma and malignant brenner tumour was 53.43, 50.75, 45.75, 40 and 60 years respectively.
Out of total 50 cases, only six were nulliparous. Oral Contraceptives (OCPs) and ovulation inducing drug consumption was seen in 4% and 2% of cases, respectively. Positive family history was found in 3/50 cases (2/50 of serous carcinoma and 1/50 of endometrioid carcinoma).
In present study, serous carcinoma (80%) topped among all the histological variants of ovarian carcinoma followed by mucinous carcinoma (8%), endometrioid carcinoma (8%), clear cell carcinoma (2%) and malignant brenner tumour (2%) [Table/Fig-1].
Spectrum of histopathological variants of ovarian carcinoma (N=50).
| Histopathological variants | Number of cases (N) | Percentage (%) |
|---|
| Serous carcinoma | 40 | 80 |
| Mucinous carcinoma | 04 | 8 |
| Endometrioid carcinoma | 04 | 8 |
| Clear cell carcinoma | 01 | 2 |
| Malignant Brenner tumour | 01 | 2 |
| Total | 50 | 100 |
Grading of serous and endometrioid carcinoma was done according to WHO two tier and three tier grading system respectively. Serous high grade carcinoma (77.50%) was more common than serous low grade carcinoma (22.50%) and all the cases of endometrioid carcinoma were moderately differentiated (Grade 2).
In the present study, maximum rise of pretreatment serum CA-125 levels (mean) were seen in serous carcinoma (1984.305 U/mL), followed by endometrioid carcinoma (1134.48 U/mL), clear cell carcinoma (72.2 U/mL), mucinous carcinoma (69.30 U/mL) and malignant brenner tumour (26.3 U/mL) [Table/Fig-2].
Pretreatment and post-treatment serum CA-125 levels in histological variants of ovarian carcinoma.
| Groups | Type of tumour |
|---|
| Serous carcinoma | Mucinous carcinoma | Endometrioid carcinoma | Clear cell carcinoma | Malignant brenner tumour |
|---|
| Pretreatment CA-125 levels (U/mL) | No of cases (n) | 40 | 04 | 04 | 01 | 01 |
| Minimum | 38.60 | 29.6 | 613.6 | 72.2 | 26.3 |
| Maximum | 13362 | 173 | 2223.7 | 72.2 | 26.3 |
| Mean±SD | 1984.305±2556.48 | 69.30±69.33 | 1134.48±735.82 | 72.2 | 26.3 |
| Post-treatment CA-125 levels (U/mL) | No of cases (n) | 37(a) | 04 | 04 | 01 | 01 |
| Minimum | 2.80 | 7.5 | 4.7 | 17.13 | 18.66 |
| Maximum | 2766 | 19.76 | 91.6 | 17.13 | 18.66 |
| Mean±SD | 155.550±527.87 | 13.360±5.05 | 37.235±37.74 | 17.13 | 18.66 |
(a): 3 were expired
Among all the serous carcinomas, mean serum CA-125 levels were more in high grade serous carcinoma (2440.013 U/mL) than low grade serous carcinoma (414.644 U/mL) and the results were statistically significant (p-value <0.001, independent-samples Mann-Whitney U Test) [Table/Fig-3].
Relationship between serum levels of pretreatment CA-125 and degree of differentiation.
| Type of tumour | Degree of differentiation | Number of cases | Mean±SD of CA-125 levels (U/mL) | Median of CA-125 levels (U/mL) | *p-value |
|---|
| Serous carcinoma | Low grade | 09 | 414.644±530.91 | 137.50 | 0.0001 |
| High grade | 31 | 2440.013±2732.65 | 1326.00 |
| Endometrioid carcinoma | Well differentiated | 00 | - | - | |
| Moderately differentiated | 04 | 1134.475±735.82 | 850.30 |
| Poorly differentiated | 00 | - | - |
| Total | | 44 | | | |
*Independent-Samples Mann-Whitney U Test
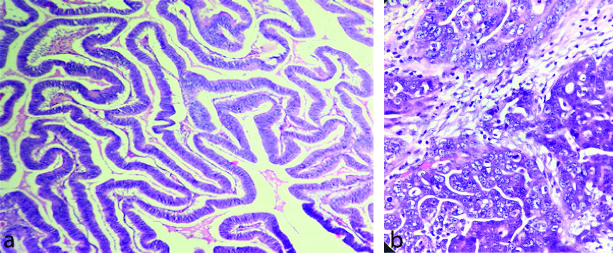
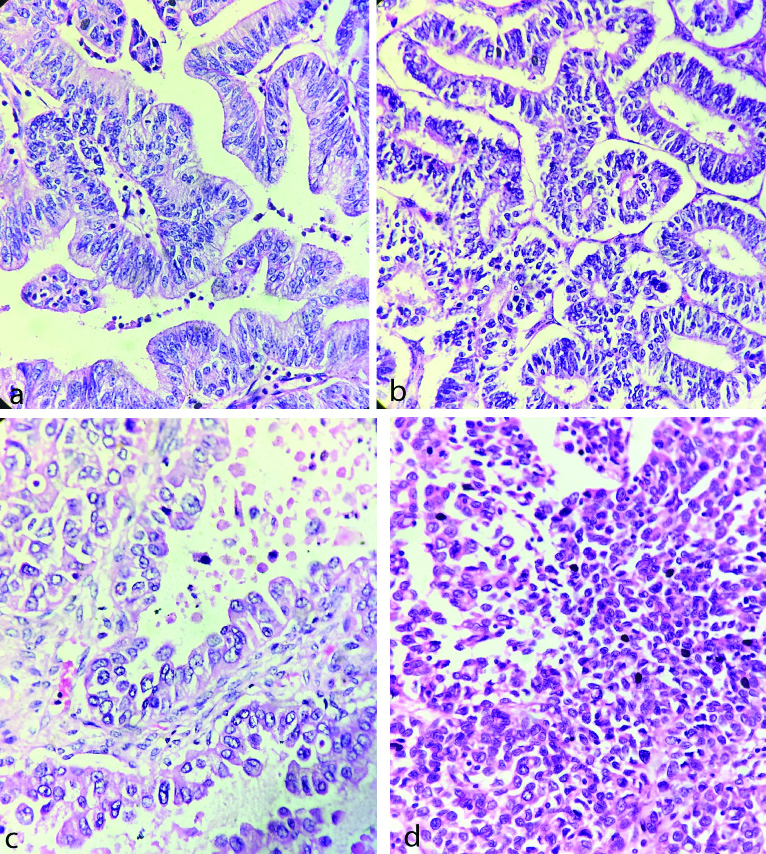
Post-treatment CA-125 levels were obtained in 47/50 cases (as 3 patients had expired). Majority (89.36%) of them had more than 50% reduction while 10.64% had either less than 50% reduction (6.38%) or an increase (4.26%) in post-treatment CA-125 levels. The histological images of low and high grade serous carcinoma is given in [Table/Fig-4a and b]. The histological changes in mucinous, endometrioid, clear cell and malignant brenner carcinomas are presented in [Table/Fig-5a, b, c and d].
a) Low grade serous carcinoma (H&E, 40X); b) High grade serous carcinoma (H&E, 40X).
a) Mucinous carcinoma; b) Endometrioid carcinoma; c) Clear cell carcinoma; and d) Malignant brenner tumour (H&E, 40X).
Discussion
The present study comprised of clinicopathological spectrum of ovarian carcinoma and its association with CA-125 levels. The study sample consisted of 50 cases of ovarian masses which were histologically diagnosed as ovarian carcinoma.
The age of presentation of ovarian carcinoma in the present study ranged from 27 to 72 years. Maximum number of the patients was seen in the age group of 41-50 years. Similar observation was made by most of the studies. (Mondal SK et al., Deeba F et al., Sarangan A et al., and Kaur S et al., [18-21].
Histologically serous carcinoma was most common in present study, comprised of total 80% of the cases, followed by mucinous carcinoma (8%), endometrioid carcinoma (8%), clear cell carcinoma (2%) and malignant brenner tumour (2%). Kaur S et al., Ahmad Z et al., and Chandanwale SS et al., conducted a study and also revealed similar results [21-23]. However, study done by Jindal D et al., 2017 finds disparity at the level of second most common malignancy and showed that clear cell carcinoma was more common than endometrioid carcinoma and mucinous carcinoma [24].
Histological grading was done as per WHO grading system and it was found that majority of serous carcinoma were high grade (77.50%), followed by low grade (22.50%) which was comparable to the studies done by Neill CJ et al., Chen M et al., and Minal J et al., [25-27].
In the present study, all the cases of endometrioid carcinoma were moderately differentiated (grade 2). This finding was in contrast to Makris GM et al., 2017 who showed that well differentiated (grade 1) endometrioid carcinoma was more common (3 cases) [28].
Serum CA-125 is an important tumour marker in ovarian carcinoma carrying diagnostic as well as prognostic significance. In the present study, mean serum CA-125 levels in pretreatment serous carcinoma were (1984.305 U/mL), endometrioid carcinoma (1134.48 U/mL), clear cell carcinoma (72.20 U/mL), mucinous carcinoma (69.30 U/mL) and in malignant brenner tumour (26.3 U/mL). Present results were similar to study done by Terzic M et al., who found that mean serum CA-125 levels were maximally raised in serous carcinoma (1245.73 U/mL), followed by endometrioid carcinoma (487.73 U/mL) and mucinous carcinoma (55.85 U/mL) [29]. In contrast Thakur V et al., 2003 found the highest mean serum CA-125 levels in endometrioid carcinoma (2853 U/mL), followed by serous carcinoma (1571 U/mL), mucinous carcinoma (775 U/mL) and clear cell carcinoma (60 U/mL). This difference was due to unequal distribution of patient within each group [30].
In the present study, pretreatment mean serum CA-125 levels. In low and high grade serous carcinoma were 414.644 U/mL and 2440.013 U/mL indicating that serum CA-125 levels were raised more in high grade than low grade serous carcinoma and the results were statistically significant (p-value=0.0001, independent-samples Mann-Whitney U Test). Present study results were concordant with Roy SK et al., and Cambruzzi E et al., who found strong association (p-value <0.001 in both) between grading and levels of CA-125 in malignant tumour, indicated by increment of CA-125 levels with higher grade of tumour [31,32]. But I et al., also showed similar results [33].
Majority of the patients (89.36%) in the present study exhibited more than 50% reduction of serum CA-125 levels after treatment, thus indicating response to therapy. However, it was noted that remaining 10.64% of the patients had either less than 50% reduction (6.38%) or increase (4.26%) in serum CA-125 levels after treatment, thus indicating static disease or disease recurrence. All these results were comparable with study done by Thakur V et al., in 2003, who evaluated that 91.4% of the cases had more than 50% reduction and 8.6% of the patients had less than 50% reduction in serum CA-125 levels post therapeutically [30].
Limitation(s)
CA-125 levels were not evaluated in benign conditions in present study. CA-125 levels rise not only in the malignant surface epithelial tumours, it also rises in various benign conditions also, thus further, studies are needed to be done.
Conclusion(s)
It is apparent from the observations of the present study that high grade serous carcinomas were most common among all the histological types of ovarian carcinoma. Higher incidence of ovarian carcinoma was seen in age group of 41-50 years. Pretreatment serum CA-125 levels were maximally raised in high grade serous carcinoma. Serum CA-125 level plays a significant role in early diagnosis of ovarian carcinoma; otherwise, most of the patients without specific symptoms would have a delay in diagnosis. Therefore, serum CA-125 level is a great tool for diagnosis, follow-up and prognosis of ovarian carcinomas.
(a): 3 were expired
*Independent-Samples Mann-Whitney U Test
[1]. Healy JC, Female reproductive system. In: Stranding S, Borley NR, Collins P, Crossman AR, Gatzoulis MA, Healy JC, et al. editorsGray’s anatomy: The anatomical basis of clinical practice 2008 40th edEdinburghElsevier Churchill Livingstone:1293-99. [Google Scholar]
[2]. D’souza B, Sreekantha D’Souza V, Hyperamylasemia in ovarian tumours- serum amylase as a marker for ovarian cancersInt J Pharm Biosci 2011 2:445-49. [Google Scholar]
[3]. Benson RC, Diagnosis and treatmentCurrent Obstet Gynaecol 1976 1:236 [Google Scholar]
[4]. Murad A, Ovulation induction and ovarian tumour: The debate continuesJ Pak Med Assoc 1998 48:353-56. [Google Scholar]
[5]. Three year report of population based cancer registries 2012-2014: Incidence, distribution, trends in incidence rates and projections of burden of cancer. [Internet]. Bengaluru, India: National centre for disease informatics and research, national cancer registry programme, and Indian Council Medical Research [cited 2016 May 2016]. Available from: http://www.ncrpindia.org/ALL_NCRP REPORTS/PBCR_ REPORT_ 2012_ 2014/ALL_ CONTEN T/Printed_Version.htm. 35 [Google Scholar]
[6]. Rajagopal L, Ravikumar U, Diagnostic utility of clinicopathological correlation in ovarian tumours: An analysis of 200 casesInt J Pharm Biosci 2015 6:1054-73. [Google Scholar]
[7]. Whittemore AS, Characteristic relating to ovarian cancer risk: Implication for prevention and detectionGynecol Oncol 1994 55:515-19.10.1006/gyno.1994.13347835800 [Google Scholar] [CrossRef] [PubMed]
[8]. Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, Livolsi VA, Clinical and pathological features of ovarian cancer in women with germline mutations of BRCA1N Engl J Med 1996 335:1413-16.10.1056/NEJM1996110733519018875917 [Google Scholar] [CrossRef] [PubMed]
[9]. Ihde DC, Longo DL, Presentations of the patient with cancer: Solid tumour in adults. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DC, editorsHarrison’s Principle of Internal Medicine 1998 14th edNew YorkMcGraw-Hill:360-62. [Google Scholar]
[10]. World Health Organisation. Classification of ovarian neoplasms. Pathology Outlines.com [Internet]. Geneva; WHO; 2016. [cited 2016 Dec 28]. Available from: http://www.Pathologyoutlines.com/topic/ovary tumortumourwhoclassif.html [Google Scholar]
[11]. Kurman RJ, Carcangiu ML, Herrington CS, Young RH, WHO classification of tumours of female reproductive organs 2014 4th edLyonIARC:11-40. [Google Scholar]
[12]. Tiwana KK, Nibhoria S, Kaur M, Monga T, Gupta R, Post-chemotherapy histopathological evaluation of ovarian carcinoma: A 40 case studyHindawi Publishing Corporation Chemother Res Prac 2015 :1-4.10.1155/2015/19787125685555 [Google Scholar] [CrossRef] [PubMed]
[13]. Gupta D, Lis CG, Role of CA-125 in predicting ovarian cancer survival- A review of the epidemiological literatureJ Ovarian Res 2009 2:1310.1186/1757-2215-2-1319818123 [Google Scholar] [CrossRef] [PubMed]
[14]. Bast RC, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC, Reactivity of a monoclonal antibody with human ovarian carcinomaJ Clin Invest 1981 68:1331-37.10.1172/JCI1103807028788 [Google Scholar] [CrossRef] [PubMed]
[15]. Gynecological Cancer Intergroup. CA-125 definitions agreed by GCIG. [Internet]. GCIG; 2005. [cited 2017, Jan 28]. Available from: http://www. gcig.igcs.org/CA-125/respdef_nov2005.pdf [Google Scholar]
[16]. Badgwell D, Bast RC, Early detection of ovarian cancerDis Markers 2007 23:397-410.10.1155/2007/30938218057523 [Google Scholar] [CrossRef] [PubMed]
[17]. Holdenrieder S, Molina R, Gion M, Gressner A, Troalen F, Auge JM, Alternative antibody for the detection of CA125 antigen: A European multicenter study for the evaluation of the analytical and clinical performance of the Access OV Monitor assay on the UniCel Dxl 800 Immunoassay SystemClin Chem Lab Med 2008 46(5):588-99.10.1515/CCLM.2008.12518598201 [Google Scholar] [CrossRef] [PubMed]
[18]. Mondal SK, Banyopadhyay R, Nag DR, Roychowdhury S, Mondal PK, Sinha SK, Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: A 10-year study in a tertiary hospital of Eastern IndiaJ Can Res Ther 2011 7:433-37.10.4103/0973-1482.9201122269405 [Google Scholar] [CrossRef] [PubMed]
[19]. Deeba F, Alam AM, Banu J, Clinlcopathological study of ovarian cancer: A multi centred studyJ Shaheed Suhrawardy Med Coll 2013 5:03-06.10.3329/jssmc.v5i1.16196 [Google Scholar] [CrossRef]
[20]. Sarangan A, Andal N, Clinicopathological and histological features of ovarian tumour- A studyISOR-JDMS 2017 16:56-60. [Google Scholar]
[21]. Kaur S, Jassal V, Bodal VK, Garg P, Kaur R, Mohi MK, A study of histological pattern of ovarian carcinoma in tertiary care Hospital PunjabInt J Curr Res Biol Med 2018 3:63-70. [Google Scholar]
[22]. Ahmad Z, Kayani N, Hasan SH, Muzaffar S, Gill MS, Histological pattern of ovarian neoplasmJ Pak Med Assoc 2001 50:416-19. [Google Scholar]
[23]. Chandanwale SS, Jadhav R, Rao R, Naragude P, Bhamnikar S, Ansari JN, Clinicopathologic study of malignant ovarian tumours: A study of fifty casesMed J DY Patil Univ 2017 10:430-37.10.4103/MJDRDYPU.MJDRDYPU_41_17 [Google Scholar] [CrossRef]
[24]. Jindal D, Sahasrabhojanee M, Jindal M, D’Souza J, Epidemiology of epithelial ovarian cancer: A tertiary hospital based study in Goa, IndiaInt J Reprod Contracept Obstet Gynecol 2017 6:2541-46.10.18203/2320-1770.ijrcog20172348 [Google Scholar] [CrossRef]
[25]. O’Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG, An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: Significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasmsAm J Surg Pathol 2005 29:1034-41.10.1097/01.pas.0000166367.68459.7d16006797 [Google Scholar] [CrossRef] [PubMed]
[26]. Chen M, Jin Y, Bi Y, Yin J, Wang Y, Pan L, A survival analysis comparing women with ovarian low-grade serous carcinoma to those with high-grade histologyOnco Targets and Therapy 2014 7:1891-99.10.2147/OTT.S6781225342912 [Google Scholar] [CrossRef] [PubMed]
[27]. Minal J, Valiathan V, Suresh PK, Sridevi HB, Grading ovarian serous carcinoma using a two tier system: Does it have prognostic significanceInt J Biomed Adv Res 2015 6:269-74.10.7439/ijbar.v6i3.1852 [Google Scholar] [CrossRef]
[28]. Makris GM, Manousopoulou G, Battista MJ, Salloum I, Chrelias G, Chrelias C, Synchronous endometrial and ovarian carcinoma: A case seriesCase Rep Oncol 2017 10(2):732-36.10.1159/00047950128878658 [Google Scholar] [CrossRef] [PubMed]
[29]. Terzic M, Dotlic J, Likic I, Nikolic B, Brndusic N, Pilic I, Diagnostic value of serum tumour markers evaluation for adnexal massesCent Eur J Med 2014 9(2):210-16.10.2478/s11536-013-0218-x [Google Scholar] [CrossRef]
[30]. Thakur V, Anand AK, Mukherjee U, Ghosh D, Determination of cancer antigen 125 in ovarian carcinomaIndian J Clin Biochem 2003 18:27-33.10.1007/BF0286736423105389 [Google Scholar] [CrossRef] [PubMed]
[31]. Roy SK, Mandal K, Mandal K, Correlation between histopathological type and grade of different ovarian tumours with their blood CA-125 LevelsJMSCR 2015 3:4078-86. [Google Scholar]
[32]. Cambruzzi E, Lima RD, Teixeira SL, Pegas KL, The relationship between serum levels of CA 125 and the degree of differentiation in ovarian neoplasmsJ Bras Patol Med Lab 2014 50:20-25.10.1590/S1676-24442014000100003 [Google Scholar] [CrossRef]
[33]. But I, Gorisek B, Preoperative value of CA-125 as a reflection of tumour grade in epithelial ovarian cancerGynecol Oncol 1996 63:166-72.10.1006/gyno.1996.03018910622 [Google Scholar] [CrossRef] [PubMed]