Introduction
The VDBP is a multifunctional protein, identified as a polymorphic protein and known as a group-specific component of serum (Gc-globulin) [1]. VDBP is a serum α2-globulin primarily responsible for the transport of vitamin D and its metabolites [2]. This multifunctional glycoprotein is a member of the albumin super family of binding proteins (albumin, α-fetoprotein and afamin). It is predominantly synthesised as a single long chain of glycoprotein in the liver [3]. Apart from its specific role in transport of vitamin D metabolites, VDBP has many biologically important functions, ranging from actin scavenging to fatty acid transport and its possible role in inflammation and in the immune system by macrophage activation [1]. VDBP is also tangled in the macrophage chemotaxis and also known to have a role in bone density. In this review, biological importance and clinical aspects of VDBP in spectra of diseases are considered with emphasis on its use as marker for diabetic nephropathy will be discussed.
Vitamin D binding protein (VDBP) was isolated from the globulin portion plasma by Hirschfeld in the year 1959 and was initially named as “group-specific component (Gc). Later the name VDBP was adopted as it was involved in binding and transportation of vitamin D metabolites. VDBP belongs to albumin superfamily, described by unique cysteine residue arrangements, with an adjoining cysteine residue distributed all over the primary structure [4]. Synthesis of VDBP is predominantly by hepatic parenchymal cell, however other tissues also produce VDBP and have been detected in plasma including other fluids like cerebrospinal fluid, seminal fluid, saliva as well as in breast milk [4].
Structure
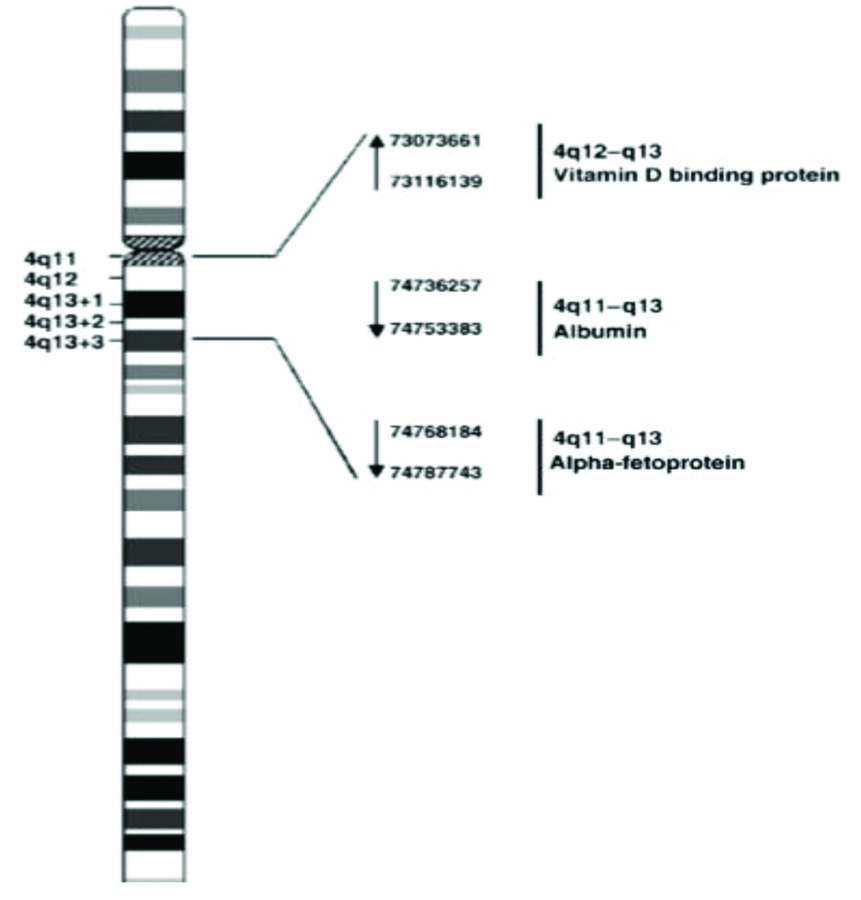
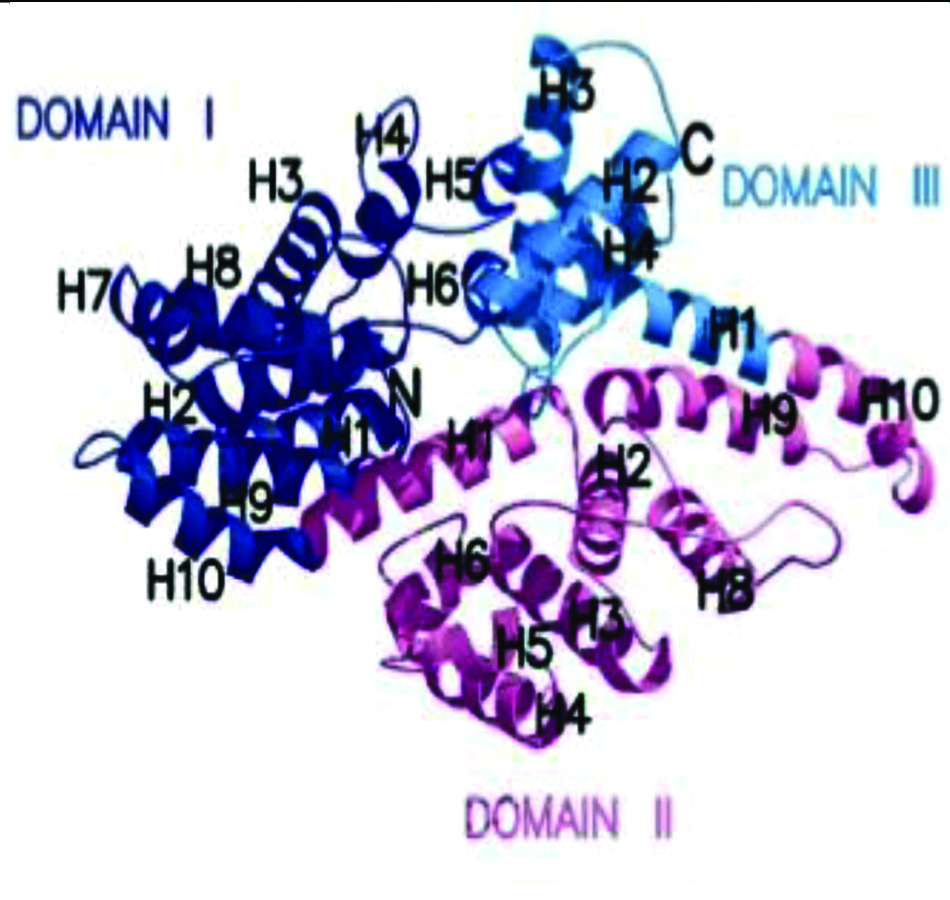
Human VDBP is encoded by VDBP gene situated on long arm of chromosome 4 (4q12-4q13) and consists of 13 exons and 12 introns and extended over 35 kb DNA. Human VDBP is composed of 458 amino acids containing numerous cysteine residues, arranged in three domains as shown in [Table/Fig-1,2] [5]. Post translational modification of VDBP is by cleavage of 16-amino-acid leader and glycosylation at N-terminal, which yields a protein with a molecular weight of 52–59 kDa [5]. Three binding domains, a vitamin D binding domain, actin binding domain have been identified between residues 35 and 49, 373 and 403 amino acid residue within VDBP sequence while a membrane binding sites identified in 150-172 and 379-402 amino acid residues [3].
Localisation of the VDBP gene on the long arm of chromosome 4 (4q12–q13) [5].
VDBP in three dimensions [8].
Using different electrophoresis methods, a considerable VDBP-polymorphism has been demonstrated in humans. More than 120 variants have been identified based on electrophoresis properties. However, three well known alleles (VDBP1F, VDBP1S and VDBP2) account for the majority of VDBP variants and are the most common. These three alleles differ by combination of two nonsynonymous Single-Nucleotide Polymorphism (SNPs) in axon 11 {rs7041 (VDBP1) and rs4588 (VDBP2)} and their glycosylation pattern as shown in [Table/Fig-3] [6]. VDBP1F and VDBP1S structure is similar besides at position 416, where aspartic acid is replaced by glutamic acid. VDBP1F and VDBP2 vary by alteration in only amino acid where threonine is replaced by lysine, whereas structure of VDBP1S and VDBP2 showed two different amino acid replacements at 416 and 420 position [6]. The two charge differences between their iso-electric point is explained by these amino acid substitutions at two different places. Mass spectrometry of a large number of patients has indicated that VDBP1 proteins are marked by an O-glycosylation on threonine at 418 and 420 whereas VDBP2 has glycosylation only at 418 [6,7].
VDBP genotype, polymorphism and amino acid residue.
| VDBP genotype | Allele at polymorphism | Amino acids at position |
|---|
| rs7041 | rs4588 | 416 | 420 |
|---|
| VDBP1F | T | C | Asp | Thr |
| VDBP1S | G | C | Glu | Thr |
| VDBP2 | T | A | Asp | Lys |
Synthesis and Turnover
A VDBP is primarily produced in the liver with an approximate production rate of 10 mg/kg/day in healthy individuals [1]. The production of VDBP follows a specific pattern of a decrease in morning and a quickelevation to a plateau through day time. VDBP has a high plasma concentration when compared with its ligand i.e., 25-hydroxyvitamin D and have a short plasma half-life of 2.5 days [7]. The plasma concentration of VDBP in healthy subjects is about 300-600 μg/mL. The VDBP maintains stable plasma concentrations throughout life with no seasonal variation and are unrelated to other plasma proteins [3]. The plasma VDBP concentration is not regulated by vitamin D sterols or any other calcitropic hormone, where as its synthesis is dependent on oestrogen and its production essentially increased with estrogen therapy as well as during pregnancy [8]. Widespread distribution of VDBP into the tissues characterises its determination in plasma, cerebrospinal fluid, saliva, breast milk and seminal fluid. The complexes of VDBP and VDBP–25-hydroxyvitamin D are eliminated from plasma by aassortment of tissues such as kidney, skeletal muscle, liver, lung, heart, bone and intestine [3]. Low plasma VDBP concentration is observed in liver diseases, nephrotic syndrome, malnutrition, septic shock, or trauma due to decrease in production or excessive protein loss [6].
Biochemical Role
Vitamin D binding protein has a number of biological functions: it functions as the carrier and reservoir for the vitamin D metabolites all over the body, along with actin scavenging, fatty acids transporter and also seems to shield the complement factor C5a from degradation by proteolysis and improve its role as a chemotactic protein [7]. VDBP also plays an important role in activation of macrophages and osteoclast.
Transport of Vitamin D Metabolites
In healthy persons, almost 85% of vitamin D metabolites in circulation is bound to VDBP whereas 15% of these metabolites are bound with albumin with lower affinity with 0.4% of total 1,25-dihydroxyvitamin D and 0.03% of total 25-hydroxyvitamin D are found in free form [9]. Vitamin D metabolites which are in bound form with VDBP have a restricted entrance to target cells making them less susceptible to metabolism in liver and resulting biliary excretion, prolonging the half-life in the circulation. The total absence of VDBP has never been demonstrated in humans, signifying that functions of VDBP may be essential to human feasibility. Affinity of VDBP for metabolites of vitamin D is fairly diverse with maximum affinity for 25-hydroxyvitamin D, trailed by 25-hydroxyvitamin D and its catabolic metabolites such as 24,25-dihydroxyvitamin D. 1,25-dihydroxyvitamin D has almost a 10 to 100 times lower affinity for VDBP than 25-hydroxyvitamin D [1].
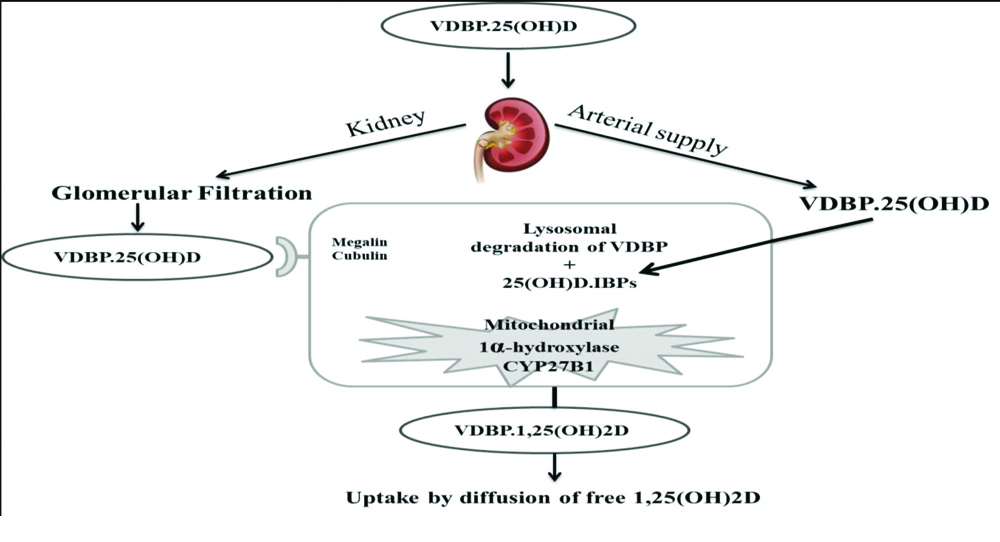
After the entry of vitamin D into general circulation, it is bound with its major carrier protein VDBP and to a lesser extent with albumin and is then subjected to hydroxylation step by the enzyme 25-hydroxylase (CYP2R1) [10], in the liver which result in formation of 25-hydroxyvitamin D. Less than 5% of 25-hydroxyvitamin D is secreted into the bile, whereas bulk of 25-hydroxyvitamin D re-enters the circulation. Once again this metabolite is bound with either VDBP or albumin for its endocrine transport to the kidney where another hydroxylation reaction takes place [10]. VDBP bound metabolites and VDBP are sieved through glomerulus and is reabsorbed by proximal tubular cells through endocytic receptor megalin as represented in [Table/Fig-4] [1]. In the kidney 25-hydroxyvitamin D is freed from its carrier protein and is subjected for further hydroxylation reaction forming 1,25-dihydroxyvitamin D and non biologically active form 24,25-dihydroxyvitamin D by the enzymes 1-alpha-hydroxylase (CYP27B1) and 24- hyxdoxylase (CYP24A1), respectively [11]. Endocytosis of VDBP-bound 25 hydroxyvitamin D mediated by megalin seems to be key pathway to reserve circulating 25-hydroxyvitamin D and to activate 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D [12].
Renal uptake of 25(OH)D by Proximal Tubular Cells (PTC) [1].
Actin Scavenging
Actin is a very much conserved cytoskeletal element of all eukaryotic cells and play important role in muscle contraction. Other essential function of actin is to maintain cell shape and motility. Globular monomeric (G-actin) and filamentous polymeric (F-actin) form are the two molecular forms of actin. After severe cell injury such as trauma, shock, sepsis, liver trauma [13], acute lung injury, large quantities of actin are released into the systemic circulation from the damaged cells. G-actin polymerises into F-actin filaments leading to an increase in blood viscosity finally leading to disseminated intravascular coagulation and multi-organ failure if it is not clear from the circulation [13]. VDBP and Gelsolin being a component of “Actin-Scavenger System” play a crucial role in the clearance of these actin filaments from the circulation [14]. Gelsolin help to depolymerise F-actin to G-actin and VDBP with high affinity for G-actin prevents the repolymerisation and finally clear it from the circulation. The clearance of VDBP-actin complex from circulation is mainly by the liver, lungs and spleen with a half-life of blood approximately 30 minutes as these tissues have receptors for the VDBP-actin complexes [13].
Fatty Acid Binding and Transport
VDBP play a contributory role in fatty acid transport by binding with mainly monounsaturated and saturated fatty acids with low affinity. The major primary role of fatty acid transport is played by albumin with higher binding capacity and is more abundant in serum indicating limited role of VDBP in fatty acid transport. Monounsaturated and polyunsaturated fatty acids is believed to decrease the affinity of 25-hydroxyvitamin D and 1,25 dihydroxyvitamin D for VDBP, whereas this is not affected by saturated fatty acids [15].
Inflammatory Process and Chemotaxis Role
The directional movement of cells as a result of an extracellular cue as a chemical gradient is chemotaxis which has been carried out with a wide variety of chemo attractants. Following inflammation, neutrophils are attracted by chemotaxis to the site of inflammation. Binding of VDBP during inflammation with complement component 5a (C5a) enhances the C5a-mediated neutrophil and macrophage chemotaxis [16]. VDBP by itself does not promote chemotaxis, nor does the VDBP-actin complex [17]. The interaction with C5a involves residues 130-149 of VDBP, a region that is common to all major VDBP alleles [16]. Vitamin D metabolites i.e., 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D is believe to be function as inhibiting factors for chemotaxis as these metabolites compete for the same binding site on VDBP [18].
Macrophage Activation
It has been demonstrated that VDBP can act as a Macrophage-Activating Factor (MAF) as it has been documented [19]. This is also supported by further analysis which revealed that activation of macrophages occurs when VDBP, a glycosylated protein was deglycosylated by T- and B-cell glycosidases to VDBP-MAF [20]. VDBP-MAF-activated macrophages demonstrate significant tumouricidal activity which is explained by its role in the immune response to neoplasia [20]. It has been also demonstrated that VDBP-MAF stimulate bone resorptive activities of osteoclasts invitro, as a flaw in lymphocytic β-galactosidase and/or some other deficit in VDBP-MAF generation which might be engaged with the pathogenesis of some osteopetrosis conditions [21]. Therapy with VDBP-MAF can partly correct the skeletal defects in osteopetrosis expanding the quantity of osteoclasts and by amending their structure [22].
Analytical Method for Estimation of VDBP
Estimation of VDBP can be done by using immunological techniques such as turbidometry, nephelometry, Enzyme-linked Immunosorbent Assay (ELISA), radioimmunoassay, rocket immunoelectrophoresis, crossed immunoelectrophoresis and single radial immunoelectrophoresis [3,23]. Using inhibition ELISA with polyclonal or monoclonal antibodies, the total VDBP can also be measured [24]. Among all the above mentioned methods, immunonephlometry offers the benefit to combine ease of use with high sensitivity and specificity and short assay time. Radioimmunoassay is more sensitive with a detection limit of 1-10 ng when compared to radioimmunodiffusion assay having a detection limit of 0.2-0.8 μg. Thus radioimmunoassay can be used as a good alternative whenever the concentration of VDBP is less than the detection limit of radioimmunodiffusion. However, the serum VDBP level is relatively high and thus radioimmunoduffusion can be used as a routine assay method [3]. The concentration of VDBP is not significantly dependent on the anticoagulant used during sample collection. As the hepatic synthesis of VDBP is oestrogen dependent, the serum level significantly increased during pregnancy and oestrogen therapy [8], whereas Low plasma VDBP concentration is observed in liver diseases, nephrotic syndrome, malnutrition, septic shock, or trauma due to decrease in production or excessive protein loss [6].
Role of VDBP on Spectra of Diseases
Cancer
Although, the basic mechanism by which vitamin D affects cancer risk is unknown, 1,25-dihydroxyvitamin D modulates cell proliferation and differentiation of both normal as well as malignant cells. It has been demonstrated that inflammatory modulation effect of 1,25-dihydroxyvitamin D or VDBP-MAF may play an important role in pathogenesis of cancer [25]. VDBP being the main carrier protein for vitamin D, has an impact on vitamin D status on cancer. Simultaneous evaluation of VDBP and 25-hydroxyvitamin D may be important in determining the association of vitamin D with cancer risk. Higher serum VDBP concentration may sequester more 25-hydroxyvitamin D and reduces free 25-hydroxyvitamin D leading to increased risk. This is showed by a recent study on pancreatic cancer [26].
Liver Disease
The level of VDBP and albumin are reduced in liver diseases which are associated with impaired protein synthesis such as cirrhosis of liver and acute liver failure [27]. Studies have documented that when compared with healthy controls and nonliver diseases, serum levels of actin-free VDBP are decreased in patients with fibrogenic liver disease and hepatocellular carcinoma. This reduction is a result of reduced de novo hepatic synthesis of this protein and correlates with the severity of disease and may be caused by the replacement of functional liver parenchyma by connective tissue [28].
Diabetes Mellitus
Type 1 diabetes patients have been found to have lower serum VDBP levels, which may indicate itself directly or indirectly to the autoimmune destruction of pancreatic β-cells [29]. VDBP affects glucose metabolism by modulating the action of vitamin D metabolites. Low plasma 25-hydroxyvitamin D levels are associated with an increased risk of type 2 diabetes, while 1,25-dihydroxyvitamin D enhances the insulin sensitivity [30,31].
Bone Disease
Variation in VDBP activity can contribute to the pathological progress of osteoporosis by the regulation of calcium metabolism in blood and bone. The major functions of VDBP in bone remodelling are nonsterol binding capacity and the macrophage/osteoclast activating activity [32]. An inverse correlation between serum VDBP levels and bone mineral density has been demonstrated, modifying the relationship between free or bioavailable 25-hydroxyvitamin D and bone mineral density in humans [33]. Study also revealed that Low serum VDBP level might be associated with a more severe character of primary hyperparathyroidism with low total 25-hydroxyvitamin D levels [34].
Kidney Diseases
Nephrotic syndrome, acute tubular necrosis, acute renal failure, CKD associated with renal tubular necrosis may have decreased transport capacity for VDBP from the glomerular filtrate into the renal tubules. Pervious study on nephrotic patients showed a lower total of free 1,25-dihydroxyvitamin D when compared with people having normal renal function [35]. Drug-induced Acute Kidney Injury (AKI) in rat model results in increased urinary loss of VDBP which is mainly caused by alteration in the sieving properties of the glomerular filtration barriers [36]. Study also demonstrated that participant with AKI had lower level of 1,25-dihydroxyvitamin D and VDBP when compared with control subjects [37]. Recent documents do not support the hypothesis that urinary loss of VDBP adds to deficiency of vitamin D in proteinuric patients. Numerous factors may account for the lack of relationship between changes in circulating vitamin D and urinary VDBP. As the tenancy of circulating VDBP by vitamin D metabolites is usually lesser than 5%, only substantial VDBP loss could leads to 25-hydroxyvitamin D deficiency or else urinary VDBP loss is normally compensated by the hepatic synthesis. Extreme nephrotic syndrome condition (i.e., proteinuria >10 g/24 hour), may add to bring down plasma VDBP levels, despite the fact that it stays improbable that this will influence plasma vitamin D levels [38].
Therapeutic USE of VDBP
The VDBP can be used in life threatening condition such as trauma, liver failure, and sepsis to reduce the patient’s risk of developing multiple organ failure due to massive actin release. A recombinant approach could open up the possibility of producing VDBP analogs with altered binding affinities or enhanced biological activities [39]. VDBP as VDBP-MAF provides protection against pulmonary deterioration thus can be useful in the treatment of chronic obstructive pulmonary diseases, as VDBP and gelsolin binds and clear the actin released from the dead cells and having capacity of increasing the viscosity of the respiratory fluid promoting airway obstruction [40]. Studies conducted by Yamamoto N et al., have published promising results with VDBP-MAF replacement therapy in cancer, and other immunesuppressed conditions, although a direct prove of the antiproliferative activity of VDBP-MAF has not yet been fully elucidated [41-44].
VDBP as A Marker for Diabetic Nephropathy
Diabetic Nephropathy (DN) is the most common microvascular complexity of diabetes mellitus, portrayed by urinary albumin excretion and/or accompanied by a slow deterioration in GFR and raised arterial blood pressure [45]. DN is a leading cause of morbidity and mortality in T2DM. It is aadvanced kidney disease caused by glomerular as well as tubular structural and functional alteration which is induced by disturbance in glucose homeostasis accounting to 30-40% of diabetics and is one of significant causes of End Stage Renal Disease (ESRD) [46].
Elevated urinary biomarkers levels can be observed in type 2 diabetic patients before the beginning of albuminuria and might be utilised as an early marker of renal injury in DN which would assume a critical part for the compelling administration and treatment approaches in diabetic consideration. There is a lacuna of knowledge with respect to the novel biomarkers which have made it from bench to bedside in recent years which can predict the future renal status and how those renal markers are better than the traditional markers. The clinical need for an ideal diagnostic and prognostic marker is still unmet setting the healthcare providers adrift in their efforts to predict which diabetic patients will progress from incipient nephropathy to overt nephropathy. uVDBP as a biomarkers in urine for detecting early DN would be ideal as this fluid is easily obtained in relatively large quantities using noninvasive procedures.
Early changes in DN include glomerular hyperfiltration, epithelial hypertrophy of glomerular and tubular cells and microalbuminuria development. These are trailed by glomerular basement membrane thickening, mesangial matrix accumulation and overt proteinuria, ultimately causing glomerulosclerosis and finally resulting in ESRD. Availability of capillary surface area for filtration decreases with the accumulation of matrix in the mesangial area and contributes to the progressive loss of renal function [47].
During kidney injury elevated excretion of various particles through urine might be brought about by either expanded tubular secretion or reduced proximal tubular reabsorption. In normal kidney, the glomerulus filter the VDBP as it is low molecular weight protein as a complex of 25-hydroxyvitamin D and VDBP which is taken up by the megalin receptors in the brush border of PTCs. Inside the cell, the complex is separated and the VDBP is degraded in lysosomes of proximal tubular epithelial cells reducing urinary excretion of VDBP to trace amount [48]. Chronic hyperglycaemia induces non enzymatic process to generate reversible glycosylation product initially and later irreversible Advance Glycation end Products (AGEs) [49].
In DN, hyperglycaemia increases Reactive Oxygen Species (ROS) and Transforming Growth Factor (TGF) production and induces inflammatory cytokines secretion (IL-18) from the podocytes causing renal damage with destruction of megalin/cubilin receptors in the proximal tubular epithelial cells, resulting in gross loss of VDBP through urine. Also, factors involved in the development of DN could regulate the receptor-mediated endocytosis, participating in enhanced urinary VDBP (uVDBP) excretion [50,51]. Further the potential role of VDBP as a noninvasive marker for early detection of DN needs to be established.
Study conducted by Tian XQ et al., observed uVDBP is significantly higher in DN with microalbuminuria and macroalbuminuria than in DM patients without albuminuria. They also found a significant difference between microalbuminuria and macroalbuminuria patients. A strong positive correlation between the expression levels of uVDBP and development of DN was also observed [52]. Mirkovic K et al., demonstrated that urinary excretion of VDBP was increased with increasing severity of renal damage and responded well to renoprotective therapy suggesting that uVDBP could be developed into a noninvasive urinary marker to monitor tubulointerstitial inflammation and fibrosis [53]. Study done by Chaykovska L et al., demonstrated that urinary VDBP is increased by four fold in diabetic patients with normoalbuminuria. These findings suggested that uVDBP is a predictor of early diagnosis of asymptomatic CKD [54].
Study conducted by Saleh Sheet MM et al., observed that uVDBP levels were significantly elevated in DN patients and positively associated with higher HbA1C and uACR and concluded that uVDBP can be used as an early predictor for the detection of DN which may help in prevention of the early onset of DN. The authors have concluded that VDBP can be used as an early marker for the diagnosis of DN [55]. Shoukry A et al., also showed that a strong positive correlation (p-value <0.001) between uVDBP and uACR where uVDBP levels were directly proportional with increased uACR levels [56].
Fawzy et al., reported uVDBP levels are exceptionally raised in Saudi patients with DN and associated essentially with severity of DN and further concluded that uVDBP could be used in combination with other conventional biomarker for early DN diagnosis and help in early diagnosis of DN and prevention of ESRD progression [57].
Limitations of VDBP as a marker of diabetic nephropathy need to be validated further. Patients with low molecular weight proteinuria secondary to multiple myeloma-associated Fanconi’s syndrome also had significant uVDBP. uVDBP is also observed to be elevated in bladder cancers and pelvic inflammatory diseases. Elevated uVDBP is also documented in population exposed to environmental pollutant such as cadmium which was linked to renal and tubular dysfunction and possibly bone lesion in patients with cadmium intoxication. The interference of vitamin D deficiency and insufficiency on the urinary clearance of VDBP should also be considered.
Conclusion(s)
Vitamin D binding protein is a multifunctional plasma protein with a wide variety of functions including Vitamin D metabolites transport, bone development, binding and transport of fatty acid, actin scavenging along with a less distinct role in immune modulation and inflammatory response. The role of VDBP in protection of microcirculation, infection, inflammation, tumour progress and octeoclast activation is still unclear. However, partially deglycosylated VDBP i.e., VDBP-MAF have shown to have antiviral and antitumoural activity and this feature of VDBP needs to be clearly elucidated. Various pathological and physiological conditions alters VDBP levels and influence vitamin D status. Liver disease reduces VDBP synthesis. Nephrotic syndrome results in excessive urinary loss while pregnancy leads to elevation of VDBP levels.
The urinary excretion of VDBP is elevated early after renal damage and is related with inflammation of tubulointerstitial cells and fibrosis independently of albuminuria. Urinary excretion of VDBP increases with increased severity of renal damage suggesting that urinary VDBP could be implicated in a combination with other conventional biomarker for the early prediction of DN and improves early diagnosis and help in prevention of progression of ESRD. This review highlights the superficial part of iceberg and spectrum of diseases in which VDBP has been implicated. However, this emerging molecule is gaining momentum as an early management marker of diabetic nephropathy.
[1]. Bouillon R, Pauwels S, The vitamin D binding protein. In: Davis Feldman editorsVitamin D 2018 4th edScience Direct: Elsevier:97-115.10.1016/B978-0-12-809965-0.00007-0 [Google Scholar] [CrossRef]
[2]. Bhan I, Vitamin D binding protein and bone healthInternational Journal of Endocrinology 2014 2014:56121410.1155/2014/56121424987416 [Google Scholar] [CrossRef] [PubMed]
[3]. Speeckaert M, Huang G, Delanghe JR, Taes Y, Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphismClinica Chimica Acta 2006 372:33-42.10.1016/j.cca.2006.03.01116697362 [Google Scholar] [CrossRef] [PubMed]
[4]. Sabetisoofyani A, Vitamin D-Binding Protein; Role in Osteoporosis. In: Ronald Ross Watson, Victor R Preedy, editorsBioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases 2013 Science Direct: Elsevier:271-77.10.1016/B978-0-12-397156-2.00019-3 [Google Scholar] [CrossRef]
[5]. Chun RF, Shieh A, Gottlieb C, Yacoubian V, Wang J, Hewison M, Vitamin D Binding Protein and the Biological Activity of Vitamin DFront Endocrinol 2019 10(718):01-15.10.3389/fendo.2019.0071831708871 [Google Scholar] [CrossRef] [PubMed]
[6]. Speeckaert MM, Speeckaert R, Van Geel N, Delanghe JR, Vitamin D binding proteinAdvances in Clinical Chemistry 2014 63:01-57.10.1016/B978-0-12-800094-6.00001-724783350 [Google Scholar] [CrossRef] [PubMed]
[7]. Chun RF, New perspectives on the vitamin D binding proteinCell Biochemistry and Function 2012 30:445-56.10.1002/cbf.283522528806 [Google Scholar] [CrossRef] [PubMed]
[8]. Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, De Ranter C, A structural basis for the unique binding features of the human vitamin D-binding proteinNat Struct Mol Biol 2002 9:131-36.10.1038/nsb75411799400 [Google Scholar] [CrossRef] [PubMed]
[9]. Armas LAG, Hollis BW, Heaney RP, Vitamin D2 is much less effective than vitamin D3 in humansJ Clin Endocrinol Metab 2004 89:5387-91.10.1210/jc.2004-036015531486 [Google Scholar] [CrossRef] [PubMed]
[10]. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW, Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylaseProc Natl Acad Sci 2004 101:7711-15.10.1073/pnas.040249010115128933 [Google Scholar] [CrossRef] [PubMed]
[11]. Jones G, Prosser DE, Kaufmann M, 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin DArch Biochem Biophys 2012 523:09-18.10.1016/j.abb.2011.11.00322100522 [Google Scholar] [CrossRef] [PubMed]
[12]. Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3Cell 1999 96:507-15.10.1016/S0092-8674(00)80655-8 [Google Scholar] [CrossRef]
[13]. Meier U, Gressner O, Lammert F, Gressner AM, Gc-globulin: Roles in response to injuryClin Chem 2006 52:1247-53.10.1373/clinchem.2005.06568016709624 [Google Scholar] [CrossRef] [PubMed]
[14]. Gressner OA, Schifflers MC, Kim P, Heuts L, Lahme B, Gressner AM, Questioning the role of actinfree Gc-Globulin as actin scavenger in neurodegenerative central nervous system disease: relationship to S-100B levels and blood-brain barrier functionClin Chim Acta 2009 400(1-2):86-90.10.1097/MEG.0b013e328329376919322100 [Google Scholar] [CrossRef] [PubMed]
[15]. Swamy N, Ray R, Fatty acid-binding site environments of serum vitamin D-binding protein and albumin are differentBioorg Chem 2008 36:165-68.10.1016/j.bioorg.2008.02.00218374965 [Google Scholar] [CrossRef] [PubMed]
[16]. Zhang J, Kew RR, Identification of a region in the vitamin D-binding protein that mediates its C5a chemotactic cofactor functionJ Biol Chem 2004 279:53282-87.10.1074/jbc.M41146220015485893 [Google Scholar] [CrossRef] [PubMed]
[17]. Ge L, Trujillo G, Miller EJ, Kew RR, Circulating complexes of the vitamin D binding protein with G-actin induce lung inflammation by targeting endothelial cellsImmunobiology 2014 219:198-207.10.1016/j.imbio.2013.10.00124268110 [Google Scholar] [CrossRef] [PubMed]
[18]. Raymond MA, Desormeaux A, Labelle A, Soulez M, Soulez G, Langelier Y, Endothelial stress induces the release of vitamin D-binding protein, a novel growth factorBiochem Biophys Res Commun 2005 338:1374-82.10.1016/j.bbrc.2005.10.10516269129 [Google Scholar] [CrossRef] [PubMed]
[19]. Nagasawa H, Uto Y, Sasaki H, Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activityAnticancer Res 2005 25:3689-95. [Google Scholar]
[20]. Yamamoto N, Urade M, Therapeutic efficacy of Gc protein derived macrophage activating factor for adenocarcinomaProc Am Assoc Cancer Res 2004 27(6):S5710.1097/00002371-200411000-00204 [Google Scholar] [CrossRef]
[21]. Swamy N, Ghosh S, Schneider GB, Ray R, Baculovirus-expressed vitamin D-binding protein-macrophage activating factor (VDBP-maf) activates osteoclasts and binding of 25-hydroxyvitamin D-3 does not influence this activityJ Cell Biochem 2001 81:535-46.10.1002/1097-4644(20010601)81:3<535::AID-JCB1067>3.0.CO;2-6 [Google Scholar] [CrossRef]
[22]. Schneider GB, Grecco KJ, Safadi FF, Popoff SN, The anabolic effects of vitamin D-binding protein-macrophage activating factor (VDBP-MAF) and a novel small peptide on boneCrit Rev Eukaryot Gene Expr 2003 13:277-84.10.1615/CritRevEukaryotGeneExpr.v13.i24.19014696974 [Google Scholar] [CrossRef] [PubMed]
[23]. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Vitamin D-binding protein and vitamin D status of black Americans and white AmericansN Engl J Med 2013 369:1991-2000.10.1056/NEJMoa130635724256378 [Google Scholar] [CrossRef] [PubMed]
[24]. Jorgensen CS, Christiansen M, Norgaard-Pedersen B, Ostergaard E, Schiodt FV, Laursen I, Gc globulin (vitamin D-binding protein) levels: An inhibition ELISA assay for determination of the total concentration of Gc globulin in plasma and serumScand J Clin Lab Invest 2004 64:157-66.10.1080/0036551041000114915115254 [Google Scholar] [CrossRef] [PubMed]
[25]. Malik S, Fu L, Juras DJ, Karmali M, Wong BY, Gozdzik A, Common variants of the vitamin D binding protein gene and adverse health outcomesCrit Rev Clin Lab Sci 2013 50:01-22.10.3109/10408363.2012.75026223427793 [Google Scholar] [CrossRef] [PubMed]
[26]. Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D, Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: A nested case–control studyCancer Res 2012 72:1190-98.10.1158/0008-5472.CAN-11-295022232734 [Google Scholar] [CrossRef] [PubMed]
[27]. Schwartz JB, Gallagher JC, Jorde R, Berg V, Walsh J, Eastell R, Determination of free 25(OH)D concentrations and their relationships to total 25(OH)D in multiple clinical populationsJ Clin Endocrinol Metab 2018 103:3278-88.10.1210/jc.2018-0029529955795 [Google Scholar] [CrossRef] [PubMed]
[28]. Gressner OA, Gao C, Siluschek M, Kim P, Gressner MA, Inverse association between serum concentrations of actin-free vitamin D-binding protein and the histopathological extent of fibrogenic liver disease or hepatocellular carcinomaEur J Gastroenterol Hepatol 2009 21:990-95.10.1097/MEG.0b013e328329376919322100 [Google Scholar] [CrossRef] [PubMed]
[29]. Blanton D, Han Z, Bierschenk L, Linga-Reddy MV, Wang H, Clare-Salzler M, Reduced serum vitamin D-binding protein levels are associated with type 1 diabetesDiabetes 2011 60:2566-70.10.2337/db11-057621844098 [Google Scholar] [CrossRef] [PubMed]
[30]. Afzal S, Bojesen SE, Nordestgaard BG, Low 25-hydroxyvitamin d and risk of type 2 diabetes: A prospective cohort study and metaanalysisClin Chem 2013 59:381-91.10.1373/clinchem.2012.19300323232064 [Google Scholar] [CrossRef] [PubMed]
[31]. Teegarden D, Donkin SS, Vitamin D: Emerging new roles in insulin sensitivityNutr Res Rev 2009 22:82-92.10.1017/S095442240938930119555519 [Google Scholar] [CrossRef] [PubMed]
[32]. Fang Y, Van Meurs JV, Arp P, Van Leeuwen JP, Hofman A, Pols HA, Vitamin D binding protein genotype and osteoporosisCalcif Tissue Int 2009 85:85-93.10.1007/s00223-009-9251-919488670 [Google Scholar] [CrossRef] [PubMed]
[33]. Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Vitamin D-binding protein modifies the vitamin D-bone mineral density relationshipJ Bone Miner Res 2011 7:1609-16.10.1002/jbmr.38721416506 [Google Scholar] [CrossRef] [PubMed]
[34]. Wang X, Shapses SA, Wei S, Sukumar D, Ghosh J, Vitamin D binding protein levels in female patients with primary hyperparathyroidismEndocr Pract 2013 19:609-13.10.4158/EP12371.OR23425651 [Google Scholar] [CrossRef] [PubMed]
[35]. Van Hoof HJ, De Sevaux RG, Van Baelen H, Swinkels LM, Klipping C, Ross HA, Relationship between free and total 1,25-dihydroxyvitamin D in conditions of modified bindingEur J Endocrinol 2001 144:391-96.10.1530/eje.0.144039111275949 [Google Scholar] [CrossRef] [PubMed]
[36]. Vicente-Vicente L, Ferreira L, Gonza lez-Buitrago JM, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI, Increased urinary excretion of albumin, hemopexin, transferrin and VVDBP correlates with chronic sensitization to gentamicin nephrotoxicity in ratsToxicology 2013 304:83-91.10.1016/j.tox.2012.12.00623261757 [Google Scholar] [CrossRef] [PubMed]
[37]. Leaf DE, Waikar SS, Wolf M, Cremers S, Bhan I, Stern L, Dysregulated mineral metabolism in patients with acute kidney injury and risk of adverse outcomesClin Endocrinol (Oxf) 2013 79:491-98.10.1111/cen.1217223414198 [Google Scholar] [CrossRef] [PubMed]
[38]. Doorenbos CR, De Cuba MM, Vogt L, Kema IP, Van den Born J, Gans RO, Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney diseaseJ Steroid Biochem Mol Biol 2012 128:56-61.10.1016/j.jsbmb.2011.09.00221958677 [Google Scholar] [CrossRef] [PubMed]
[39]. Gomme PT, Bertolini J, Therapeutic potential of vitamin D-binding proteinTrends Biotechnol 2004 22:340-45.10.1016/j.tibtech.2004.05.00115245906 [Google Scholar] [CrossRef] [PubMed]
[40]. Schellenberg D, Pare PD, Weir TD, Spinelli JJ, Walker BA, Sandford AJ, Vitamin D binding protein variants and the risk of COPDAm J Respir Crit Care Med 1998 157:957-61.10.1164/ajrccm.157.3.97061069517617 [Google Scholar] [CrossRef] [PubMed]
[41]. Yamamoto N, Suyama H, Nakazato H, Yamamoto N, Koga Y, Immunotherapy of metastatic colorectal cancer with vitamin D-binding protein-derived macrophageactivating factor, GcMAFCancer Immunol Immunother 2008 57:1007-16.10.1007/s00262-007-0431-z18058096 [Google Scholar] [CrossRef] [PubMed]
[42]. Yamamoto N, Suyama H, Nakazato H, Yamamoto N, Ushijima N, Immunotherapy of metastatic breast cancer patients with vitamin D-binding protein-derived macrophage activating factor (GcMAF)Int J Cancer 2008 122:461-67.10.1002/ijc.2310717935130 [Google Scholar] [CrossRef] [PubMed]
[43]. Yamamoto N, Suyama H, Immunotherapy for prostate cancer with Gc protein derived macrophage-activating factor, GcMAFTransl Oncol 2008 1:65-72.10.1593/tlo.0810618633461 [Google Scholar] [CrossRef] [PubMed]
[44]. Yamamoto N, Ushijima N, Koga Y, Immunotherapy of HIV-infected patients with Gc protein-derived macrophage activating factor (GcMAF)J Med Virol 2009 81:16-26.10.1002/jmv.2137619031451 [Google Scholar] [CrossRef] [PubMed]
[45]. Ni X, Gu Y, Yu H, Wang S, Chen Y, Wang X, Serum adipocyte fatty acid-binding protein 4 levels are independently associated with radioisotope glomerular filtration rate in type 2 diabetic patients with early diabetic nephropathyBioMed Research International 2018 2018:457814010.1155/2018/457814029992142 [Google Scholar] [CrossRef] [PubMed]
[46]. Sharma RK, Diabetes and kidney disease. In: YP Munjal, editorsAPI Textbook of Medicine 2015 110th editionJaypee Brothers Medical Publisher:528-33. [Google Scholar]
[47]. Nazar CMJ, Diabetic nephropathy; principles of diagnosis and treatment of diabetic kidney diseaseJ Nephropharmacol 2014 3(1):15-20. [Google Scholar]
[48]. Mahadevappa R, Nielsen R, Christensen EI, Birn H, Megalin in acute kidney injury foe and friendAmerican Journal of Physiology Renal Physiology 2014 306:147-54.10.1152/ajprenal.00378.201324197071 [Google Scholar] [CrossRef] [PubMed]
[49]. Singh AK, Mo W, Dunea G, Arruda JA, Effect of glycated proteins on the matrix of glomerular epithelial cellsJ Am Soc Nephrol 1998 9:802-10.10.1681/ASN.V958029596077 [Google Scholar] [CrossRef] [PubMed]
[50]. Satirapoj B, Adler SG, Prevalence and Management of Diabetic Nephropathy in Western CountriesKidney Dis 2015 1:61-70.10.1159/00038202827536666 [Google Scholar] [CrossRef] [PubMed]
[51]. Heilig CW, Deb DK, Abdul A, Riaz H, James LR, Salameh J, GLUT1 regulation of the pro-sclerotic mediators of diabetic nephropathyAm J Nephrol 2013 38:39-49.10.1159/00035198923817135 [Google Scholar] [CrossRef] [PubMed]
[52]. Tian XQ, Zhao LM, Ge JP, Zhang Y, Xu YC, Elevated urinary level of vitamin D-binding protein as a novel biomarker for diabetic nephropathyExp Ther Med 2014 7:411-16.10.3892/etm.2013.142624396416 [Google Scholar] [CrossRef] [PubMed]
[53]. Mirkovic K, Doorenbos CR, Dam WA, Lambers Heerspink HJ, Slagman MC, Nauta FL, Urinary vitamin D binding protein: A potential novel marker of renal interstitial inflammation and fibrosisPLoS One 2013 8(2):01-09.10.1371/journal.pone.005588723409077 [Google Scholar] [CrossRef] [PubMed]
[54]. Chaykovska L, Heunisch F, Einem G, Alter ML, Hocher CF, Tsuprykov O, Urinary Vitamin D binding protein and KIM-1 are potent new biomarkers of major adverse renal events in patients undergoing coronary angiographyPLoS One 2016 11:1-11.10.1371/journal.pone.014572326751954 [Google Scholar] [CrossRef] [PubMed]
[55]. Saleh Sheet MM, AL-karawi INS, Alkhafajy HSA, Urinary Vitamin D binding protein as an early predictor of diabetic nephropathy in type 1 and type 2 diabetesIbn Al-Haitham Jour For Pure and Appl Sci 2018 31:15-22.10.30526/31.1.1845 [Google Scholar] [CrossRef]
[56]. Shoukry A, Bdeer SEA, El-Sokkary RH, Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabeticnephropathy in type 2 diabetes mellitusMolecular & Cellular Biochemistry 2015 408:25-35.10.1007/s11010-015-2479-y26104579 [Google Scholar] [CrossRef] [PubMed]
[57]. Fawzy MS, Abu AlSel BT, Assesment of Vitamin D binding protein and early prediction of nephropathy in type 2 Saudi diabetic patientsJournal of Diabetes Research 2018 2018:851792910.1155/2018/851792929850609 [Google Scholar] [CrossRef] [PubMed]