Caesarean Scar Pregnancy (CSP) is a rare entity. Gestational Trophoblastic Disease (GTD) in a CSP is exceedingly rare. This can lead to complications like rupture uterus, severe haemorrhage, hypovolemia which may require hysterectomy, endangering a woman’s life, her future fertility and death. As no therapeutic protocols have been established about this rare condition, it is difficult to diagnose and manage. The case report describes a patient, 26-year-old gravida 2 para 1, diagnosed with a CSP with partial mole. She was treated with systemic Methotrexate (MTX) followed by surgery-wedge resection of ectopic mass and repair of uterus. Patient remained on β-hCG follow-up. The management of CSP requires high clinical suspicion and immediate action with combination of various treatment modalities. Primary caesarean section rate must be reduced by performing caesareans only for justified reasons, so as to decrease the incidence of scar pregnancies.
Case Report
A 26-year-old gravida 2 para 1 patient presented to us in Out Patient Department (OPD) with history of bleeding per vaginum and pain abdomen for last one and half months. She was a case of previous caesarean section done four years back, with secondary infertility for which she was undergoing investigation and treatment from a private clinic. One and a half months back she had excessive bleeding per vaginum with passage of clots after overdue by three days. For this she visited some private clinic where her urine pregnancy test was found to be positive and ultrasonography revealed retained products of conception. She was prescribed tablet Misoprostol 800 μg and was called for follow-up. The excessive bleeding per vaginum continued for three days, for which suction and evacuation was done in the same clinic. Minimal to heavy bleeding per vaginum continued for another two weeks. She visited another private clinic where suction and evacuation was done twice in view of incomplete abortion. No uterotonic drugs were used at the time of suction and evacuation. However, there was no relief in symptoms and the patient was referred to our institution.
General physical examination and vitals were within normal limits. There was a well healed Pfannensteil scar on abdominal examination. On per speculum examination minimal bleeding through cervical os was present and per vaginum examination revealed a bulky soft uterus with fornices clear. Patient was admitted and investigated. The same day serum β-hCG was 29427.1 mIU/mL and ultrasonography done by radiologist was suggestive of CSP of size 3.5×3 cm with Endometrial Thickness (ET) 4.2 mm. Bilateral ovaries were normal and there was no free fluid in pouch of Douglas [Table/Fig-1]. Magnetic Resonance Imaging (MRI) was done next day showing possibility of either molar pregnancy or scar ectopic [Table/Fig-2]. Chest X-ray and thyroid function tests were normal. Serum β-hCG after 48 hours was 30138.9 mIU/mL. Patient was decided for conservative management. Injection MTX 75 mg intramuscular was given and vitals were monitored. Repeat β-hCG on day four and seven of injection MTX was 37933.8 mIU/mL and 30900.2 mIU/mL respectively. Follow-up ultrasonography on day seven showed CSP of size 3.9×3.0 cm with ET of 4.7 mm. Despite of fall in β-hCG levels on day 7 of injection MTX, the size of caesarean pregnancy was increasing. Hence, the patient was decided for exploratory laparotomy.
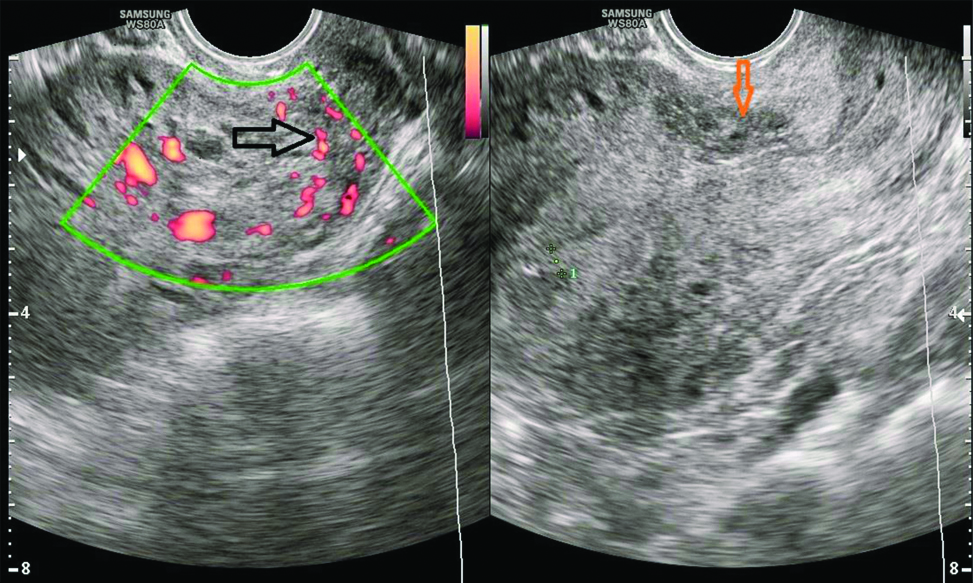
Transvaginal Sonography (TVS)-Heterogenously hyperechoic mass 3.5×3 cm in caesarean scar towards right side with increased vascularity (Black arrow shows increased vascularity on Doppler, Orange arrow shows caesarean scar site with pregnancy of size 3.5×3 cm).
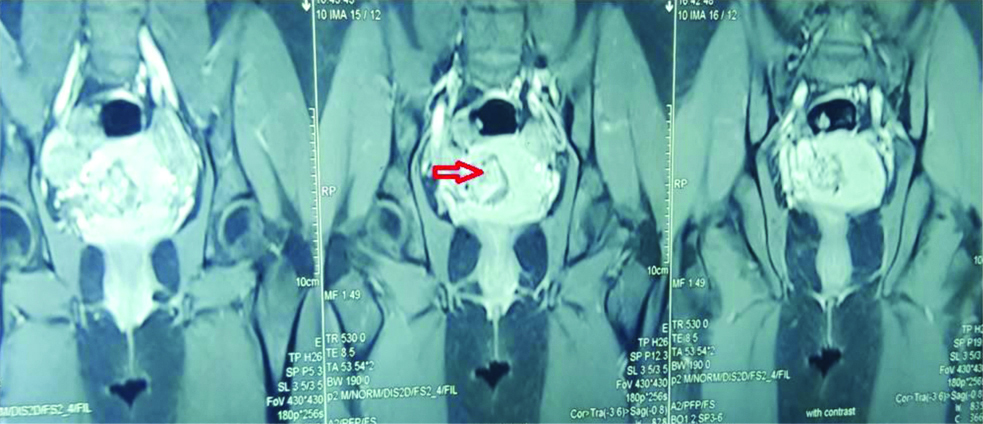
MRI-Coronal view (T1 weighted, post contrast)- Heterogenously hyperenhancing mass 3.8×3.7 cm in LUS with post contrast enhancement (red arrow).
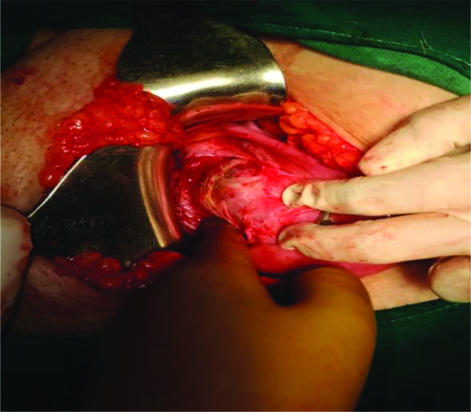
On laparotomy Lower Uterine Segment (LUS) was thinned out with bluish mass of size 4×6 cm bulging towards right side with serosa intact, suggestive of ectopic pregnancy with scar dehiscence [Table/Fig-3]. On incising the scar site, a mass of 4×6 cm size was removed having placental bits, blood clots and few vesicles in it [Table/Fig-4]. Wedge resection of mass along with myometrium was done and followed by repair of the uterus. Bilateral tubes and ovaries were grossly normal. Approximate blood loss was 300 mL. The specimen was sent for Histopathological Examination (HPE).
Intraoperative- Bluish mass 4×6 cm bulging towards right side with thinned out caesarean scar with serosa intact suggestive of ectopic pregnancy with scar dehiscence.
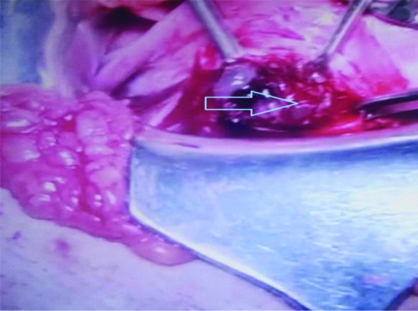
Intraoperative- 4×6 cm mass at caesarean scar with placental bits and few vesicles (arrow)
The β-hCG on first Postoperative Day (POD) was 5075 mIU/mL and on third POD was 1749.9 mIU/mL. Patient was discharged on third POD with an uneventful healing and adviced review with HPE report and weekly follow-up with serum β-hCG. The HPE of specimen was suggestive of partial hydatiform mole in CSP [Table/Fig-5]. Patient remained on weekly β-hCG follow-up which became less than 5 mIU/mL after eight weeks and negative for next three consecutive weeks.
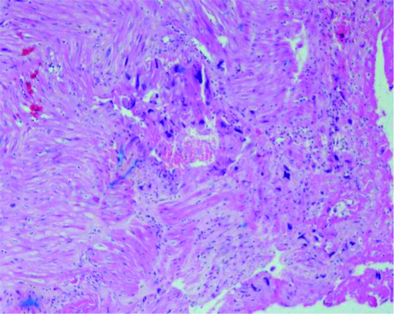
Histopathology (low power 10X, H&E staining) - Varying size of villi, focal trophoblastic hyperplasia and atypia and scalloping of chorionic villi.
Discussion
The CSP is a rare entity. The first case of CSP was reported by Larsen and Solomon in 1978 [1]. There is a rising trend in number of cases being reported possibly due to increasing prevalence of caesarean sections and increasing use of better imaging studies [2]. The frequency of CSP is reported to be 1:1800 to 1:2226 (0.05-0.04%) of all pregnancies [2,3]. In a woman after caesarean section the frequency of CSP is approximately 0.15% [3]. The GTD in a CSP is very rare. Only few cases have been reported till now.
The CSP is defined as the implantation of a gestational sac within the scar of a previous caesarean surgery. The exact cause of CSP is not clear. It is presumed that there is an early invasion of the myometrium through a microscopic tract in the caesarean section scar tissue. In CSP, the gestational sac gets embedded within the fibrous tissue of the previous caesarean section scar. Trauma incurred during other uterine surgeries like manual removal of placenta, uterine curettage, myomectomy, hysteroscopy can also lead to formation of such tracts and subsequently CSP. The relationship between number of caesarean sections and risk of CSP has not been established. In a recent systematic review, it was found that 52%, 36% and 12% cases followed one, two and three or more previous caesarean sections [4].
CSP can be classified into two types-Endogenous and exogenous. Endogenous CSP is caused by implantation of gestational sac into caesarean scar defect with growth towards uterine cavity whereas in exogenous CSP growth infiltrates into uterine myometrium and bulges into uterine serosa and bladder [5].
The CSP is rare, however molar pregnancy occurring in scar is even rarer. On reviewing literature, we could only find very few cases of CSP with GTDs. The first case reported was a partial molar pregnancy in caesarean scar described by Wu CF et al., in 2006 [6]. The patient had Dilatation and Curettage (D&C) in view of partial molar pregnancy based on ultrasound findings and high serum β-hCG values; histological test revealed incomplete hydatidiform mole. About 7 days after first D&C, there was persistent vaginal bleeding, high β-hCG levels (30.756 IU/L) and vaginal ultrasound showed vascularised residual tissue on the caesarean scar, hence second D&C was undertaken. The β-hCG level gradually went back to normal and spotting disappeared in nine weeks without serious complications [6].
In the year 2008, Sukumaran S et al., reported a case at nine weeks who presented with painless vaginal bleeding. On work-up her serum β-hCG was found to be 25,779 IU/l and ultrasound scan demonstrated a mass at the site of previous caesarean section with increased flow on colour doppler. Molar pregnancy in caesarean scar was diagnosed and MRI confirmed the diagnosis. MTX was used for the treatment. They concluded that ultrasound could be used as a vital tool for the diagnosis of CSP [7].
Another case of molar pregnancy on previous hysterotomy scar was reported by Michener C and Dickinson JE in 2009. The patient was treated with MTX injection into the gestational sac. Urgent hysterectomy was performed due to severe intractable vaginal haemorrhage. Histological test confirmed molar pregnancy [8].
Jin FS et al., in 2011 reported a case in which the ultrasound of a patient showed a gestational sac located at the site of a scar from a previous caesarean section. She had presented with irregular vaginal bleeding. The patient underwent suction curettage and the subsequent pathology report revealed partial mole [9].
Ko JK et al., described a case in 2012 where the patient presented after failed attempt at surgical evacuation of pregnancy. Ultrasound-guided D&C followed by uterine artery embolisation was performed. Histological test indicated partial hydatidiform mole [10].
Kaluarachchi C et al., reported a case of a multiparous female at nine weeks of gestation where β hCG was rising without an intrauterine pregnancy. Two laparoscopies were done followed by a laparotomy when caesarean site pregnancy was suspected. They performed a subtotal hysterectomy. A hydatidiform (H) mole was confirmed on HPE [11].
In a case report by Dagdeviren EG et al., a 34-year-old patient with previous caesarean section was diagnosed ultrasonographically as having molar pregnancy in caesarean scar. Her human chorionic gonadotropin level was measured as 59.705 mIU/mL. As there was risk of rigorous bleeding, caesarean section scar excision were done via laparotomy. The pathology outcome was complete molar pregnancy, so the patient was followed up according to molar pregnancy follow-up protocols and cured entirely [12].
A case of complete molar pregnancy in a caesarean scar with myometrial infiltration was reported by Badia V et al., [13]. After two attempts of D&C and persistent vaginal bleeding, high β-hCG values and presence of caesarean scar vascular mass located on ultrasonography, hysterectomy was performed. Histopathology indicated invasive H mole [13].
Jiang HR et al., reported a case of amenorrhoea with vaginal bleeding. Ultrasound revealed a mass at caesarean site with high β hCG. Suction evacuation resulted in excessive bleeding which was controlled by uterine artery embolisation. The HPE confirmed H mole. Chemotherapy was also given in view of persistence of mass [14].
Early diagnosis and adequate treatment are crucial for maternal health and preservation of fertility [15]. High clinical suspicion especially after failed surgical evacuations is very important. Our patient underwent repeated curettage and finally excision of scar tissue was done. Similar findings have been described in literature. Delay in diagnosis can lead to catastrophic complications like rupture uterus, severe haemorrhage which may even require hysterectomy.
The TVS is thought to be the best and first line diagnostic tool. Diagnostic criteria are: An empty uterine cavity and an empty cervical canal, a gestational sac in anterior part of uterine isthmus, an absence of healthy myometrium between the bladder and the gestational sac and circular blood flow surrounding the sac must also be clearly visible. Doppler examination is essential for correct diagnosis because it shows vascularisation around the caesarean scar. It shows functional placental vascularisation caused by increased blood flow with peak systolic velocity greater than 20 cm/s and pulsatility index lower than 1. The MRI may be reserved for cases where there is a diagnostic problem. It helps in precise dimension of distance between the urinary bladder, myometrium and gestational sac and provide good quality visualization of the uterine cavity and cervical canal [16].
In addition, molar pregnancy in caesarean scar is detected by initial abnormally elevated serum β-hCG levels, persistence of symptoms after primary treatment, increase in volume of ectopic mass and vascularisation and cystic changes on ultrasound [13].
Various management strategies range from conservative management to radical surgery. Different modalities tried in literature include injection MTX via different routes, suction curettage preferably under ultrasound guidance, endoscopic (laparoscopic and hysteroscopic) CSP excision, laparotomy to excise and repair the CSP site, uterine artery embolisation in combination with other treatment modalities, intracervical injection of vasopressin prior to uterine evacuation of CSP and high intensity focused ultrasound combined with suction curettage under hysteroscopic guidance, hysterectomy, etc. Treatments should be individualised based on specific characteristics of each CSP, its imaging features and patient preferences. Systemic MTX seems to be most effective for CSP with β-hCG below 5000 IU/mL whereas in CSP with β-hCG levels above 10,000 IU/mL simultaneous systemic and local MTX may be a very effective method [4]. For better therapeutic effects multistep treatment like systemic MTX followed by ultrasound guided/hysteroscopic guided suction curettage or uterine artery embolisation followed by suction curettage, insertion and inflation of foley balloon catheter to prevent or stop bleeding, can be used. Hysterotomy is used in more advanced CSP cases as well as in uterine rupture and massive haemorrhage. Wedge resection followed by repair of uterus is done to protect fertility. Women managed expectantly will mostly develop placenta accrete or increta resulting in either a hysterotomy or hysterectomy with severe haemorrhage [17].
The GTD in CSP is very rare. As there are no therapeutic protocols, so it is difficult to diagnose and manage. Jin FS et al., in their case report of partial mole in CSP did ultrasound guided suction and curettage with serum β-hCG follow-up thereafter [9]. In similar case report by Wu CF et al., patient underwent ultrasound guided suction and curettage twice week apart with serum β-hCG monitoring [6]. In case report of Badia V et al., of complete molar pregnancy in caesarean scar with myometrial infiltration the patient landed up in hysterectomy [13]. In our case, patient had suction and evacuation thrice for misdiagnosed incomplete abortion followed by conservative management with systemic MTX (75 mg i.m). However, wedge resection of CSP with repair of uterus in order to prevent catastrophic complication and preserve her future fertility was done.
The incidence of CSP is due to increasing caesarean sections. A primary caesarean scar invariably invites repeat scars and possibly more CSP. Therefore, as a preventive measure it would be important to monitor a primary labour and perform justified caesarean section.
Conclusion(s)
Although a molar pregnancy in caesarean scar is very difficult to diagnose, the initial abnormally elevated serum β-hCG levels, persistence of symptoms after primary treatment, increase in volume of ectopic mass and vascularisation and cystic changes despite the drop in β-hCG levels may be useful criteria for diagnosis. Timely and appropriate treatment with individualised approach and patient preferences is to be done to prevent life threatening complications.
[1]. Larsen JV, Solomon MH, Pregnancy in uterine scar sacculus-An unusual case of post abortal haemorrhage. A Case reportS Afr Med J 1978 53(4):142-43. [Google Scholar]
[2]. Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ, First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scarUltrasound Obstet Gynecol 2003 21(3):220-27.10.1002/uog.5612666214 [Google Scholar] [CrossRef] [PubMed]
[3]. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL, Cesarean scar pregnancy: Issues in managementUltrasound Obstet Gynecol 2004 23(3):247-53.10.1002/uog.97415027012 [Google Scholar] [CrossRef] [PubMed]
[4]. Rotas MA, Haberman S, Levgur M, Caesarean scar ectopic pregnancies: Etiology, diagnosis, and managementObstet Gynecol 2006 107(6):1373-81.10.1097/01.AOG.0000218690.24494.ce16738166 [Google Scholar] [CrossRef] [PubMed]
[5]. Vial Y, Petignat P, Hohlfeld P, Pregnancy in a cesarean scarUltrasound Obstet Gynecol 2000 16(6):592-93.10.1046/j.1469-0705.2000.00300-2.x11169360 [Google Scholar] [CrossRef] [PubMed]
[6]. Wu CF, Hsu CY, Chen CP, Ectopic molar pregnancy in a caesarean scar: A case reportTaiwanese J Obstet Gynecol 2006 45(4):343-45.10.1016/S1028-4559(09)60257-6 [Google Scholar] [CrossRef]
[7]. Sukumaran S, Duckett RA, Evans M, Molar pregnancy in caesarean scar- A case report and the role of ultrasound as a diagnostic tool Obstetrics & GynaecologyPresented at 18th World Congress on Ultrasound in Obstetrics and Gynecology, held in Chicago, USA on August 2008 [Google Scholar]
[8]. Michener C, Dickinson JE, Caesarean scar ectopic pregnancy: A single centre case seriesAust N Z J Obstet Gynaecol 2009 49:451-55.10.1111/j.1479-828X.2009.01067.x19780724 [Google Scholar] [CrossRef] [PubMed]
[9]. Jin FS, Ding DC, Wu GJ, Hwang KS, Molar pregnancy in a cesarean section scar of uterusJournal of Medical Sciences 2011 31(4):173-76. [Google Scholar]
[10]. Ko JK, Wan HL, Ngu SF, Cheung VY, Ng EH, Cesarean scar molar pregnancyObstet Gynecol 2012 119:449-51.10.1097/AOG.0b013e3182322f3122270435 [Google Scholar] [CrossRef] [PubMed]
[11]. Kaluarachchi C, Tissera A, Gananatha Karunarathna S, Caesarean scar site complete molar pregnancySri Lanka Journal of Obstetrics and Gynaecology 2013 35(2):62-64.10.4038/sljog.v35i2.6162 [Google Scholar] [CrossRef]
[12]. Dağdeviren EG, Dur R, Fadıloğlu E, Demirdağ E, Öztürk Ç, Altay M, Molar pregnancy in cesarean section scar: A case reportTurk J Obstet Gynecol 2017 14(4):249-51. [Google Scholar]
[13]. Badia V, Torcia F, Scarani S, Maniglio P, Catalano A, Caserta D, Gestational trophoblastic neoplasia arising on scar pregnancy: A case reportClin Case Rep Int 2018 2:1035 [Google Scholar]
[14]. Jiang HR, Shi WW, Liang X, Zhang H, Tan Y, Hydatidiform mole in a scar on the uterus: A case reportWorld J Clin Cases 2020 8(8):1547-53.10.12998/wjcc.v8.i8.154732368549 [Google Scholar] [CrossRef] [PubMed]
[15]. Maymon R, Halperin R, Mendlovic S, Schneider D, Vaknin Z, Herman A, Ectopic pregnancies in Caesarean section scars: The 8 year experience of one medical centreHum Reprod 2004 19(2):278-84.10.1093/humrep/deh06014747167 [Google Scholar] [CrossRef] [PubMed]
[16]. Hoffman T, Lin J, Cesarean scar ectopic pregnancy: Diagnosis with ultrasoundClin Pract Cases Emerg Med 2020 4(1):65-68.Published 2020 Jan 1510.5811/cpcem.2019.10.4398832064429 [Google Scholar] [CrossRef] [PubMed]
[17]. Malik MF, Hoyos LR, Rodriguez-Kovacs J, Gillum J, Johnson SC, Placenta increta complicating persistent cesarean scar ectopic pregnancy following failed excision with subsequent preterm cesarean hysterectomyCase Reports in Obstetrics and Gynecology 2016 2016:40718405 pages, 201610.1155/2016/407184027375911 [Google Scholar] [CrossRef] [PubMed]