Introduction
Cefepime, a fourth generation cephalosporin antibiotic, is primarily used in hospitalised patients. It is predominantly cleared via the renal system, yet its neurotoxic effects have been reported chiefly in patients with impaired renal function. Several predisposing conditions such as impaired renal function, dysfunction of the Blood Brain Barrier (BBB) and ϓ Aminobutyric Acid (GABA) antagonism are responsible for the neurotoxic effects [1,2]. In majority of the cases, cefepime induced neurotoxicity are reversible after discontinuation of the drug [3].
Recognising these neurotoxic events to be drug related in critically ill patients is a challenge, as it is assumed to be deterioration of the disease, which further delays and increases the toxic effects. Below is a series of cases of cefepime induced neurotoxicity in critically ill patients. The intent of this article was to demonstrate the varied manifestation of the clinical symptoms, probable risk factors responsible for the toxicity, duration of onset for development and regression of symptoms, management and patient outcome.
Case Series
Case 1
A 63-year-old female presented with bilateral swelling of legs, decreased urinary output and shortness of breath on exertion. Patient had a history of Chronic Kidney Disease (CKD), Type II Diabetes Mellitus (T2DM) (since 15 years) and secondary hypertension (15 years).
Clinical examination was suggestive of pedal oedema, mild pallor, with decreased breath sounds on auscultation. The serum creatinine was 2.9 mg/dL. Her chest X-ray revealed bilateral pleural effusion. Blood pressure was 160/90 mmHg and random blood sugar was 220 mg/dL. Her 2D Echocardiography (2D Echo) suggested global left ventricular hypokinesia (ejection fraction 29%). Patient was started with Intravenous (IV) cefepime 1 gm and tazobactam 125 mg every 8 hourly along with IV furosemide 20 mg 12 hourly.
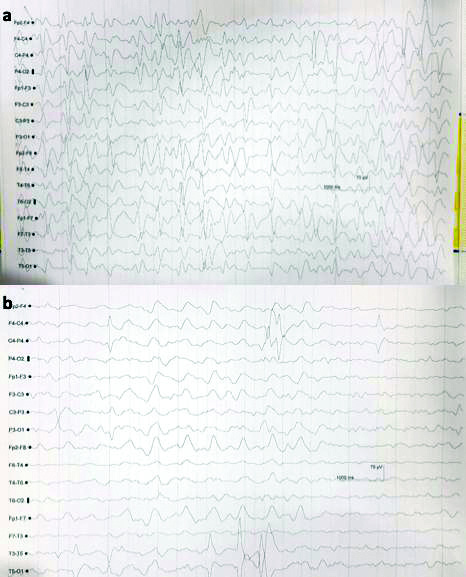
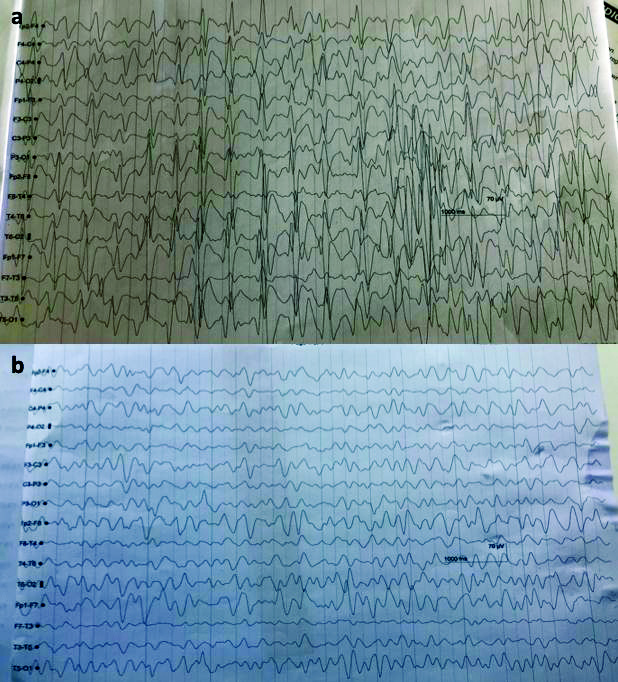
Nine days after starting cefepime, patient became drowsy, disoriented and irritable with Glasgow Coma Scale (GCS) of 10. A Non Contrast Computed Tomography (NCCT) scan of head was normal and Electroencephalogram (EEG) showed generalised spike and waves epileptiform discharges seen with frequency of >2.5 Hz and persists for >10 secs in a epoch, suggestive of non convulsive epilepsy [Table/Fig-1a]. She was treated with antiseizure medication like levetiracetam, midazolam infusion. There was no episode of seizure and a repeat EEG was taken [Table/Fig-1b]. However there was no improvement in sensorium. Later, it was suspected that cefepime could be the cause for neurotoxicity and it was withdrawn on 19thday. She improved after withdrawal of cefepime. Her sensorium started improving but subsequently she developed catheter induced urinary tract infection and refractory septic shock which lead to her death on 30th day.
a) EEG showed Generalised spike and waves triphasic waves with frequency >2.5 Hz, persists for >10 secs in a epoch, suggestive of non convulsive epilepsy and b) Repeat EEG showing diffuse slowing of background suggestive of encephalopathy.
Case 2
A 62-year-old male was hospitalised for complaints of shortness of breath, nausea, vomiting and profuse perspiration. Patient had a medical history of End Stage Renal Disease (ESRD) with dialysis (twice weekly from six months), T2DM (15 years), and secondary hypertension (15 years). He had stroke with right thalamic bleed two years back and Dilated Cardiomyopathy (DCM) four months back. Patients also had a history of left-sided hemiparesis with altered sensorium. Laboratory investigations showed white blood cells were 11,300/cumm, serum creatinine was 13.6 mg/dL and 15-20 pus cells in urine sample. His chest X-ray showed signs of fluid overload and 2D Echo revealed global left ventricular hypokinesia with moderate dysfunction (ejection fraction 36%), mild mitral and tricuspid regurgitation and pulmonary arterial hypertension.
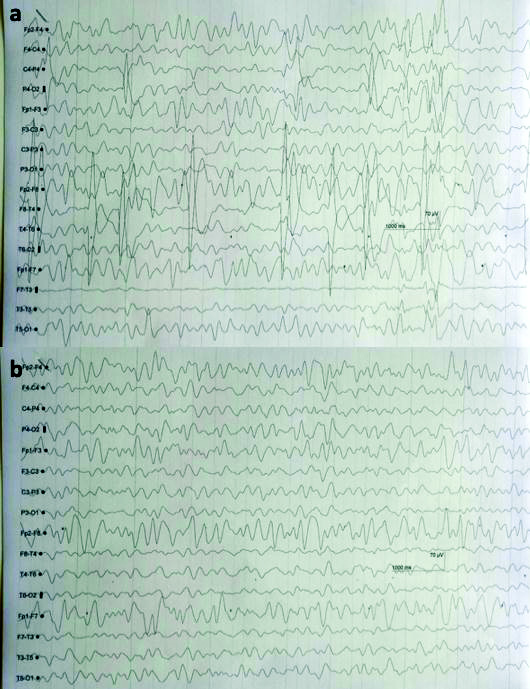
Patient was immediately started on haemodialysis with Non Invasive Ventilation (NIV) and IV cefepime 1 gm and tazobactam 125 mg every 8 hourly along with IV furosemide 40 mg 12 hourly. After four days of cefepime initiation, he developed slurred speech, tremors of both hands (left>right) and weakness of left leg. His NCCT showed right thalamic infarct and he was started on aspirin 150 mg and atorvastatin 20 mg. He was highly irritable with lowering of GCS (altered sensorium with left sided hemiparesis: GCS E3M4V3) for which he was started on IV sodium valproate 200 mg 12 hourly and injection haloperidol (2.5 mg) and promethazine 12.5 mg. His EEG on ninth day showed generalised but frontal predominant triphasic waves overlapping with slow back ground consisting of theta and delta waves suggestive of encephalopathy [Table/Fig-2a].
EEG shows a) Generalised but frontal predominant triphasic waves seen overlapping slow back ground consists of theta and delta waves suggestive of encephalopathy and b) Generalised but anterior (frontal-parietal) predominant triphasic waves were seen overlapping and slow back ground consisting of theta waves, suggestive of encephalopathy.
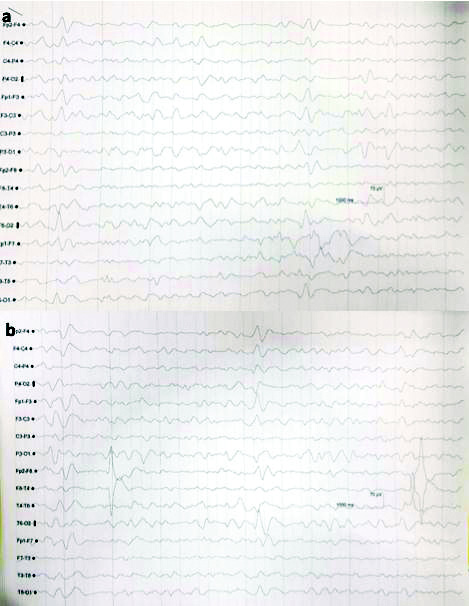
Drug related neurotoxicity of cefepime was suspected as the probable cause for it [Table/Fig-2b]. After withdrawing injection cefepime, his sensorium gradually started to improve (GCS: E4M5V4) along with normalisation of EEG changes. However, weakness of left side persisted.
Case 3
A 73-year-old male was hospitalised for complaints of shortness of breath and reduced urine output for three days along with bilateral swelling of legs for one day. On admission, the blood pressure was 219/105 mmHg with pallor and bilateral moderate pedal oedema. Laboratory investigations showed anaemia Haemoglobin (Hb) was (6.7%), serum creatinine was 7.3 mg/dL and potassium was 6.2 mEq/L. He was diagnosed with Chronic Obstructive Pulmonary Disease (COPD), diabetes mellitus, secondary hypertension and CKD. The 2D Echo showed global hypokinesia with Left Ventricular Ejection Fraction (LVEF) of 46%.
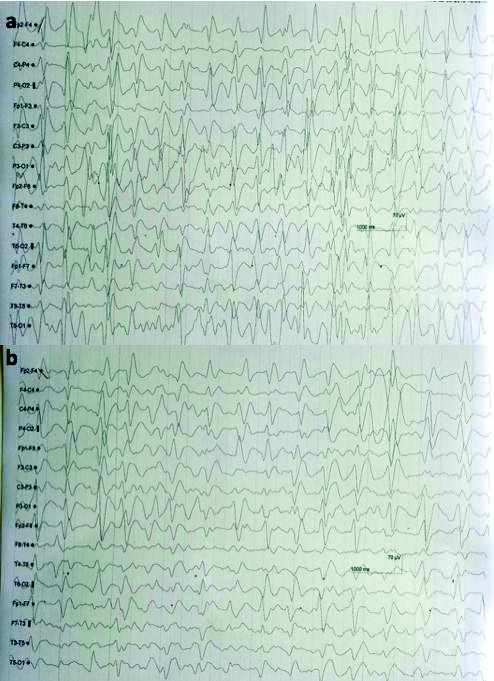
Patient was started with NIV and IV cefepime 1 gm and tazobactam 125 mg every 8 hourly and a unit of Packed Red Blood Cells (PRBC). After six days of starting cefepime, he developed drowsiness and sluggish response. His brain CT scan was normal but EEG showed generalised epileptic form spike and waves with frequency >2.5 Hz, persisting in almost all epochs throughout the recording. These findings were suggestive of Non Convulsive Status Epilepticus (NCSE) for which he was started on antiepileptic drugs [Table/Fig-3a]. The MRI brain and cerebrospinal fluid study had no significant changes. Suspecting drug induced neurotoxicity, both cefepime and tazobactam were stopped. There was a marked improvement in sensorium from day 13 and patient was discharged on day 17 [Table/Fig-3b].
EEG showed Generalised spike and waves epileptiform with frequency a) >2.5 Hz, persists in almost all epochs throughout the recording, suggestive of non convulsive epilepsy and b) 1-2 Hz, persisting in all epochs throughout the recording, suggestive of non convulsive epilepsy.
Case 4
A 61-year-old female was hospitalised with complaints of shortness of breath for one day with past medical history of CKD (for one year), T2DM and secondary hypertension (both for five years). Haemoglobin was 5.2%, serum creatinine was 3.9 mg/dL and urine examination showed signs of infection with 35-40 pus cells. Her chest X-ray showed signs of fluid overload.
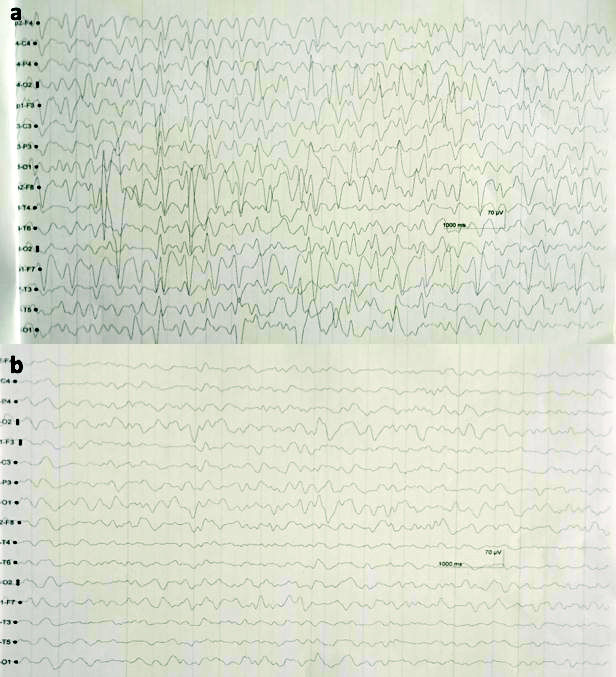
Patient was transfused with a unit of PRBC and treatment with IV cefepime 1 gm and tazobactam 125 mg every eight hourly along with IV furosemide 40 mg 12 hourly was initiated. On fifth day of hospitalisation, patient was irritable and had vomiting with one episode of seizure. Her NCCT brain failed to show any abnormalities while EEG showed continuous generalised spike and wave epileptiform discharges with frequency of 2 to 2.5 Hz persisting in most of the EEG traces [Table/Fig-4a]. This was followed by disappearance of epileptiform activity with midazolam challenge test [Table/Fig-4b]. Her MRI brain showed acute infarct in left posterior cerebral artery and right superior cerebellar artery. Suspecting drug toxicity cefepime was withdrawn. Sensorium gradually improved from day 12th and she was subsequently discharged.
The EEG shows a) Continuous generalised spike and wave epileptiform discharges with frequency of 2 to 2.5 Hz persists in most of the EEG traces and b) Disappearance of epileptiform discharges with midazolam challenge test.
Case 5
A 64-year-old female was admitted with complaints of shortness of breath and generalised weakness since one month. She had medical history of secondary hypertension and CKD with dialysis (twice a week from two months). The haemoglobin was 8.2%, serum creatinine was 7.6 mg/dL, potassium was 5.8 mEq/L and pus cells (20-22) in urine indicating urine tract infection.
Patient was started on IV cefepime 1 gm and tazobactam 125 mg every 8 hourly with other supportive care and hemodialysis. From day 4, she developed drowsiness which was followed by increased irritability and altered sensorium and delirium on day 5. Cefepime neurotoxicity was suspected hence it was stopped the same day. The next day patient developed one episode of generalised motor onset tonic-clonic seizure and her EEG showed generalised spike and waves epileptiform discharges with frequency >2.5 Hz, persisting in almost all epochs throughout the recording suggestive of non convulsive epilepsy [Table/Fig-5a]. Her NCCT brain was normal. On day 7, the EEG showed disappearance of epileptiform discharges with normalised background activity with midazolam 2 mg [Table/Fig-5b]. From day 8, her sensorium started to improve and subsequently patient was discharged with near normal state.
The EEG shows a) Generalised spike and waves epileptiform with frequency >2.5 Hz, persists in almost all epochs throughout the recording, suggestive of non convulsive epilepsy and b) Disappearance of epileptiform discharges with normalised background with the effect of injection midazolam 2 mg during the EEG test.
Case 6
A 54-year-old female with medical history of secondary hypertension, T2DM and ESRD with dialysis (twice a week from last two week) was hospitalised for compliant of generalised weakness. On examination, her vitals were deranged with blood pressure of 180/100mmHg and haemoglobin was 8.8%, serum creatinine was 6.6 mg/dL and the serum potassium were 5.7 mEq/L. She was consulted with a urologist for arteriovenous fistula.
Patient was immediately started on haemodialysis and cefepime 1 gm and tazobactam 125 mg every 8 hourly along with other supportive treatment. On day 5, she developed fever for which blood culture was advised and according to sensitivity report, piperacillin 2 gm and tazobactam 250 mg was administered while cefepime 1 gm and tazobactam 125 mg were stopped. On day 4, patient developed mild disorientation with hyperkalemia (7.1 mEq/L). She developed altered sensorium, abnormal movements like twitching of perioral area, eyebrows and myoclonic jerky movements of left upper limb and both lower limbs.
The EEG showed spike and wave epileptiform discharges with frequency of 0.5-1Hz with slow back ground (theta waves) activity [Table/Fig-6a]. She was treated with injection sodium valproate 1000 mg loading followed by 500 mg twice daily. After antiseizure medication and withdrawal of injection cefepime, her sensorium improved and she was discharged on day 9 [Table/Fig-6b].
The EEG shows a) Spike and wave epileptiform discharges with frequency of 0.5-1 Hz with slow back ground (theta waves) activity and b) Relatively normal back ground activity with occasional spike and wave epileptiform discharges.
Discussion
Cefepime is a frequently used antibiotic in hospitalised patients. It is known to cause toxicity of which its impact on the central nervous system is known to be reported scarcely. Certain predisposing factors such as impaired renal functioning, dysfunction of Blood Brain Barrier (BBB) and GABA antagonism can produce neurotoxicity.
In this case series, patients with renal failure in the Intensive Care Unit (ICU) with cefepime neurotoxicity were studied [Table/Fig-7]. Majority of the patients were of older age groups (>60 yrs) with co-morbidities such as secondary hypertension, cardiac abnormalities and diabetes mellitus. The most common clinical findings of cefepime toxicity were of drowsiness, altered sensorium, myoclonus, encephalopathy and NCSE. All the above six patients reported within a duration of two months (July 2019 to August 2019), thereby indicating the higher prevalence of cefepime toxicity but only few cases are actually reported of these neurological manifestations post cefepime administration.
Summary of findings of cefepime toxicity and its outcome.
| Case number | Age/gender | GFR (mL/min) | Dose of Cefepime | Onset of symptom from initiation (day) | EEG changes | Outcome |
|---|
| Case 1 | 63/F | 11 | 1 gm TDS | 16 | Seizure disorder with encephalopathy | Death |
| Case 2 | 62/M | <10 | 1 gm TDS | 6 | NCSE | 14 days DAMA* |
| Case 3 | 73/M | <10 | 1 gm TDS | 1 | NCSE | 15 days delirious, disoriented |
| Case 4 | 61/F | 13.7 | 1 gm TDS | 4 | Continuous generalised discharge | Vegetative discharged |
| Case 5 | 64/F | <10 | 1 gm TDS | 4 | Status epilepticus | 9 days (recovered) |
| Case 6 | 54/F | <10 | 1 gm TDS | 4 | Diffused slowing of background waves (Status epilepticus) | 7 days (recovered) |
*discharged against medical advice was due to personal reasons from patient’s family.
DAMA: Discharged against medical advice; EEG: Electroencephalogram; GFR: Glomerular filtration rate; TDS: Thrice-a-day; NCSE: Non convulsive status epilepticus
Majority of cefepime induced toxicity cases are unrecognised and under reported [4]. Owing to the incidence of neurological complications and the risk of seizure development, the Food and Drug Administration (FDA) United States in 2012 issued safety announcement for physicians to adjust the dose of cefepime in patients with renal damage [5]. This referred to 59 cases which had been reported in the FDA’s Adverse Event Reporting database with NCSE due to cefepime from 1996 till 2012.
Cefepime is a potent, fourth generation, broad spectral antibiotic commonly prescribed in critically ill patients against a majority of pathogens. Identifying the exact cause of neurotoxicity is a challenge since the clinical manifestations can mimic those of progression of the presenting disease such as metabolic dysfunction, systemic infections, hepatic failure, uraemia or diabetic acidosis. Since 85% of the drug is excreted via the kidneys, hence the risk of drug accumulation becomes high in renal dysfunction [6]. But, even reports of cefepime induced neurotoxicity in patients with normal renal functioning are presented in literature [6]. The exact mechanism for the occurrence of this neurotoxicity is not clear but it has been proposed that beta lactam group of antibiotics like cefepime competitively antagonize GABA which leads to depolarization at the post synaptic junction developing neurological symptom [7].
The manifestations of neurotoxicity post cefepime administration in ICU patients develop after 3-4 days; hence they are misinterpreted as disease symptoms causing a delay and intensification of the symptoms [8]. In most cases these symptoms are reversible after discontinuation of cefepime or after dialysis along with symptomatic management as antiepileptics [9]. The half-life of cefepime is 0.5-2.0 hours but in renal impaired patients it increases upto 13 hours thereby causing excessive exposure of the drug [10]. Hence, despite dose adjustments, the high serum value of the drug might cause neurotoxicity. In critically ill patients with impaired renal functioning who are on supportive care, calculating the exact glomerular filtration for dose adjustment can be a challenge. Due to the critical illness for which cefepime is administered; the inflammatory state produces disruption in the functioning of BBB causing excess accumulation of the drug in the nervous system.
The neurological symptoms start with altered sensorium, drowsiness, reduced consciousness, depression, confusion, disorientation, myoclonus or seizures. In patients with compromised renal impairment reducing the dose of cefepime is recommended but still several studies have been reported wherein neurotoxicity developed despite dose adjustments [11]. In a study by Fugate JE et al., 28.9% of patients developed cefepime neurotoxicity though they were on reduced dosage regime [8]. There are even reports of patients who were on haemodialysis yet they developed cefepime neurotoxicity [12]. Calculating the concentration of cefepime in serum or cerebrospinal fluid would be the most diagnostic measure to judge the neurotoxicity but such practise is not followed in routine setup.
In majority of cases, the symptoms of neurotoxicity resemble/mimic those of stroke, are often missed to be caused by cefepime toxicity. In such cases, discontinuation of the drug brings a reversal of the symptoms confirming the aetiology. Even in the present series, case 2 had right thalamic infarct while case 4 developed acute infarct in right and left cerebral artery. But on discontinuing cefepime in second case regained her sensorium and the neurological symptoms ceased while in case 4, despite discontinuation, the patient failed to revert and condition deteriorated, which could possibly be due to infarct.
According to Garces EO et al., the development of cefepime induced encephalopathy from 2005 to 2006 was related to the degree of renal impairment [13]. In his study on 498 patients administered with cefepime, five cases were diagnosed of cefepime induced encephalopathy. These patients had a mean Glomerular filtration rate (GFR) of 17.20 mL/min plus 10.75 mL/min while the patients without any symptom of encephalopathy, the mean GFR were 32.59 mL/min plus 14.89 mL/min. In present case series all six cases were with impaired renal function, with majority of cases (n=4) had GFR <10 mL/min thereby confirming the above hypothesis.
EEG is of greater aid in identifying the neural changes while suspecting drug toxicity. In a systematic review of 135 cases of cefepime induced encephalopathy, 98 cases had EEG abnormalities with 40% showing triphasic waves, 39% focal sharp waves, 25% NCSE and 7% myoclonic status epilepticus [9]. These findings were confirmed even in the present case series as EEG detected the neurotoxic changes confirming the diagnosis. The study by Payne LE et al., also stated that neurological symptoms developed on an average four days after drug initiation and resolved in two days on an average [9]. The present series showed development of these symptoms from one to six days and were resolved in 7-15 days. However, in case 1, which ended in death despite cessation of cefepime, could probably be complicated with other co-morbid conditions.
Conclusion(s)
To conclude, the aim of this case series presentation was to generate awareness amongst clinicians in treating critically ill patients with cefepime. Any neurological manifestations post cefepime administration should be confirmed by EEG or serum or cerebrospinal fluid levels of the drug and should be immediately discontinued. In patients with ESRD dose adjustment should be carefully done along with haemodialysis to minimise the risk of irreversible changes or mortality.
Cefepime, though being a potent broad spectrum antibiotic, can manifest its neurotoxic features both in patients with normal and impaired renal functioning. Since it is primarily administered in hospitalised and critically ill patients, high index of suspicion is required in patients with probable risk factors. Modification of its dose in renal function impaired cases should be considered. Clinicians should consider drug toxicity in neurological manifestation of critically ill patients. Aggressive antiepileptic treatment is required as in majority of cases discontinuation of cefepime reverses the neurological symptoms.
*discharged against medical advice was due to personal reasons from patient’s family.
DAMA: Discharged against medical advice; EEG: Electroencephalogram; GFR: Glomerular filtration rate; TDS: Thrice-a-day; NCSE: Non convulsive status epilepticus
[1]. Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V, Hary L, Maizel J, Prud’homme-Bernardy A, Blood and CSF monitoring of cefepime-induced neurotoxicity: Nine case reportsJ Antimicrob Chemother 2012 67(5):1297-99.10.1093/jac/dks01222298349 [Google Scholar] [CrossRef] [PubMed]
[2]. Cefepime [package insert] Bristol-Myers Squibb Company, Princeton, NJ; 2016. Lat Accessed February 15, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf [Google Scholar]
[3]. Lamoth F, Buclin T, Pascual A, High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal functionAntimicrob Agents Chemother 2010 54(10):4360-67.10.1128/AAC.01595-0820625153 [Google Scholar] [CrossRef] [PubMed]
[4]. Schlidt K, Kadlec A, Bhandari S, Jha P, Cefepime-induced neurotoxicity: Five cases reported in a single institutionCureus 2018 10(11):e366610.7759/cureus.366630740285 [Google Scholar] [CrossRef] [PubMed]
[5]. FDA Drug Safety Communication. Cefepime and Risk of Seizure in Patients not Receiving Dosage Adjustments for Kidney Impairment. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm309661.htm. Last Accessed February15, 2021 [Google Scholar]
[6]. Bragatti Jose A, Cefepime-induced neurotoxicityCentral Nervous System Agents in Medicinal Chemistry 2008 8(4):229-33.10.2174/187152408786848139 [Google Scholar] [CrossRef]
[7]. Meillier A, Rahimian D, Cefepime-induced encephalopathy with normal renal functionOxf Med Case Reports 2016 2016(6):118-120.10.1093/omcr/omw04227274853 [Google Scholar] [CrossRef] [PubMed]
[8]. Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA, Cefepime neurotoxicity in the intensive care unit: A cause of severe, underappreciated encephalopathyCrit Care 2013 17(6):R26410.1186/cc1309424200036 [Google Scholar] [CrossRef] [PubMed]
[9]. Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, Cefepime-induced neurotoxicity: A systematic reviewCrit Care 2017 21(1):27610.1186/s13054-017-1856-129137682 [Google Scholar] [CrossRef] [PubMed]
[10]. Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA, Pharmacokinetics of cefepime in subjects with renal insufficiencyClin Pharmacol Ther 1990 48(3):268-76.10.1038/clpt.1990.1492401125 [Google Scholar] [CrossRef] [PubMed]
[11]. Gangireddy VG, Mitchell LC, Coleman T, Cefepime neurotoxicity despite renal adjusted dosingScand J Infect Dis 2011 43(10):827-29.10.3109/00365548.2011.58130821604923 [Google Scholar] [CrossRef] [PubMed]
[12]. Yadla M, Kishore CK, Sriramnaveen P, Reddy YS, Sainaresh VV, Bhuma V, Neurotoxicity due to cefepime in patients on maintenance hemodialysisSaudi J Kidney Dis Transpl 2011 22(5):1026-27. [Google Scholar]
[13]. Garces EO, Andrade de Anzambuja MF, da Silva D, Bragatti JA, Jacoby T, Saldanha Thomé F, Renal failure is a risk factor for cefepime-induced encephalopathyJ Nephrol 2008 21(4):526-34. [Google Scholar]