Pre-eclampsia is a pregnancy specific syndrome, characterised by inflammatory responses and affecting various organ systems. Worldwide, its incidence ranges from 2-8% of all pregnancies [1]. The hallmarks of pre-eclampsia are new onset hypertension and proteinuria. Various studies mention that placental hypoxia in pre-eclampsia causes there lease of antiangiogenic factors, which in turn causes fenestration of the normal endothelium of glomerular capillaries, leading to proteinuria [2,3]. Proteinuria leads to the loss of albumin in urine. This loss cannot be compensated by hepatic production. This hypoalbuminemia is said to be an early sign of pre-eclampsia [4].
Proteinuria leads to loss of proteins other than albumin, like hormone binding globulins including Thyroid Binding Globulins (TBG) and hormones like thyroxine in urine [5]. The loss of albumin and TBG decreases total T3 and T4 concentrations. Free thyroid hormones fT3 and fT4 are also affected because the massive proteinuria may cause gastrointestinal oedema, leading to lack of absorption of synthetic materials needed for synthesising thyroid hormones [6].
It is already known that pre-eclampsia increases the risk of dyslipidaemia, heart disease and stroke in later life. Studies by Basbug M et al., and Kumar A et al., have detected hypothyroidism in pre-eclampsia [7,8]. Since, hypothyroidism leads to preterm delivery, postpartum bleeding and cardiac complications in mother and intrauterine death, congenital hypothyroidism, low Intelligence Quotient (IQ) later in life in the fetus [9], it will be beneficial to study whether hypoalbuminemia in pre-eclampsia is associated with changes in thyroid hormone levels, thereby causing hypothyroidism.
The present study is a part of a previously published study done for testing the thyroid profile (T3, T4, TSH, fT3, fT4) in pre-eclampsia [10]. In this study, aim to compare the serum albumin levels and thyroid profile and find any association between hypoalbuminemia and hypothyroidism in pre-eclampsia. This may give insight into whether hypothyroidism and its complications can be prevented by decreasing the albuminuria, by early and adequate management of pre-eclampsia.
Materials and Methods
This case-control study was done at Department of Biochemistry and the Department of Obstetrics and Gynaecology of Government Medical College, Thiruvananthapuram, Kerala, India, during a period of one year from January 17, 2012 to January 16, 2013. This study was conducted after getting the clearance from the Institutional Ethical Committee IEC no: 1/30/2012.
Sample size calculation: Study was conducted with 40 pre-eclampsia patients between 30-38 weeks of gestation as cases and 40 healthy pregnant women in third trimester as controls. Sample size was calculated using the formula:
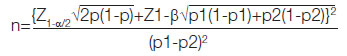
Where P=(p1+p2)/2 [11]. At 5% significance level Zα=1.96, at 80% power, Z1-β=0.842 (values of p1 and p2 was taken from a previous study) [8].
Pre-eclampsia is defined as the occurrence of hypertension (BP≥140/90 mmHg) and significant proteinuria (≥300 mg/day) in previously healthy pregnant women on or after 20th week of gestation [12].
Inclusion Criteria
For cases: All diagnosed cases of pre-eclampsia between 30-38 weeks of gestation with no previous history of thyroid disease during pregnancy and no previous history of congenitally malformed baby were included in this study.
For controls: Equal number of healthy normotensive pregnant women in third trimester attending the antenatal clinic during the study period constituted the control group were included in this study.
Exclusion Criteria
For cases: History of any metabolic disorder, including thyroid disorders before or during the pregnancy, history of intake of any medication, history of hypertension or renal disease were excluded in this study.
For controls: History of any previous thyroid disease, congenitally malformed baby, hypertension, renal disease, any metabolic disorder, or any intake of medication that might affect thyroid function were excluded in this study.
Study Procedure
Pateints details were recorded in proforma after getting written informed consent. Symptoms suggestive of severe pre-eclampsia like headache with visual disturbances, epigastric pain etc., and abnormal laboratory results like those of liver or renal function tests etc., were recorded. A 5 mL of venous blood was collected from study subjects, centrifuged and serum was stored at -20°C until use.
Measurement of serum albumin: Serum albumin was measured by ERBA liquixx albumin kit [13]. Method used was Bromocresol green method. It is an end point assay, done in fully automated ERBA XL 360 analyser [13]. Albumin forms blue green coloured complex with Bromocresol green at pH 4.2. The colour formed is directly proportional to the concentration of albumin present in the sample when measured photometrically between 580-630 nm with maximum absorbance at 620 nm.
Reagents used were albumin reagent (Bromocresol green 0.08 mmol/L, Succinate buffer 50 mmol/L, Sodium azide 1 g/L, surfactant), Albumin standard-3.6 g/dL
Pipette 1000 μL of reagent one into all three test tubes marked as blank, standard and test. Pipette distilled water 10 μL to ‘blank’ test tube alone, albumin standard 10 μL to ‘standard’ test tube alone and sample 10 μL to the test tube alone. Mix it well and read the absorbance of standard and each sample at 630 nm (580-630 nm) after one minute incubation at 37°C.
Calculation:
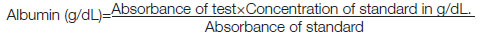
Expected range of serum albumin was 3.5-5 g/dL [13]
Measurement of thyroid profile: Thyroid profile (FT3, FT4, T3, T4 and TSH) was measured using ERBA thyrokit Enzyme Linked Immunosorbent Assay (ELISA) method following instructions in the kit literature provided along with it and read in ELx 800MS, ERBA Microscan ELISA machine.
Expected ranges for thyroid hormones were T3=0.8-2 ng/mL, T4=4.4-12.8 μg/dL, FT3=1.4-4.2 pg/mL, FT4=0.8-2 ng/dL (These are the reference ranges followed in central biochemistry lab in the government medical college in which the study was done), TSH=0.5-5 mIU/L (normal females) and 0.13-3.5 mIU/L (in third trimester of pregnancy) [10,14]. Hypoalbuminemia was taken as serum albumin <3.5 g/dL in the present study, hypothyroidism was defined as TSH >3.5 mIU/L in pregnant women [14].
Statistical Analysis
Statistical analysis was performed using Statistical Package For The Social Sciences (SPSS) version 16 (2007, Chicago, SPSS Inc). The mean and standard deviation for quantitative variables was calculated for the study population. Difference in percentage values were compared using Chi-square test or Fischer’s t-test. Difference in the group means of quantitative variables was compared by student t-test. The correlation between albumin and thyroid profile was obtained by Pearson’s correlation coefficient. Significance level was taken as p≤0.05.
Results
The mean age of normal pregnant women were 24.5±3 years and of pre-eclampsia patients were 24.98±2.8 years. The mean gestational age of controls were 35.5±2.5 weeks and that of cases were 34.5±2.6 years, This difference in age and parity between two groups was not significant. A 65% of the cases were primigravida and 57.5% of the controls were primigravida and the difference was not significant (p=0.491). Thus, the cases and the controls were comparable.
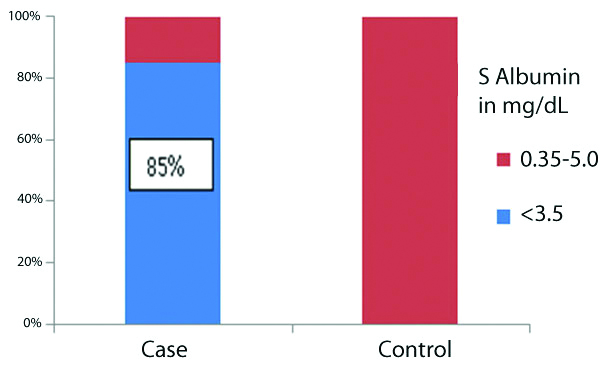
The mean albumin levels in controls (3.7±0.14 mg/dL) was significantly higher than in cases (3.11±0.39 mg/dL) with p<0.01. Among cases, 34 (85%) out of 40 had albumin below less than 3.5 g/dL none of the controls had low albumin. Fischer’s t-test was done and this was statistically significant (p<0.001). Remaining six cases (15%) had albumin within the normal range (3.5-5 g/dL). All of the controls had albumin in the normal range [Table/Fig-1]. Thus, significantly higher percentage of cases had low albumin than controls (p<0.01, Fischer’s-exact test).
Distribution of serum albumin values among cases and controls.
The mean and median T3 of cases was found to be significantly lower than controls (p<0.001). The mean and median T4 of cases was found to be lower than controls. The mean TSH levels in cases were 3.76±1.55 and in controls were 2.30±0.94 mIU/L.T4 values were seen to have wide difference between minimum and maximum values. However, 75% of preeclamptics and 33% of controls had T4 levels in the expected range (4.4-12.8 μg/dL). Neither pre-eclampsia nor normal group had T4 below expected range. The mean T3 of cases was 1.28 ng/mL which was significantly lower than controls (1.62). The mean T4 of cases was 11.59 μg/dL and controls were 13.63. The mean TSH of cases was found to be higher than controls and the difference was statistically significant (p<0.001). The mean and median values of fT3 (2.12±0.55 pg/mL) and fT4 (1.16±0.24 ng/dL) among cases was found to be significantly lower than controls.
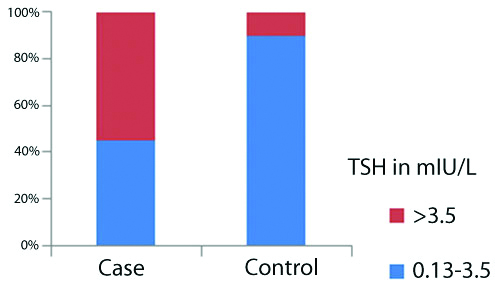
A total of 22 (55%) cases and four controls (10% of controls) had TSH values above 3.5 mIU/L. Rest had TSH values between 0.13-3.5 mIU/L [Table/Fig-2]. Chi-square test also showed p<0.05, showing significant percent difference between two groups.
Distribution of TSH values in cases and controls.
A total of 40 cases were divided into 18 severe pre-eclampsia and 22 mild pre-eclampsia patients based on severity assessment criteria [15]. All participants were grouped into Overt Hypothyroidism (OH) and Subclinical Hypothyroidism (SCH) or normal as defined in this study based on laboratory results [16]. In severe group, out of 18 patients, one had OH, 13 had SCH and four were normal. Among the 22 mild preeclamptics, one had OH, seven had SCH and the rest 14 were normal. Among control group, four have SCH and the rest 36 were normal [Table/Fig-3].
Thyroid status according to severity of pre-eclampsia.
| Thyroid status | Severe PE | Mild PE | Controls |
|---|
| N | % | N | % | N | % |
|---|
| Hypothyroidism | OH# | 1 | 5.6 | 1 | 4.6 | 0 | 0 |
| SCH* | 13 | 72.2 | 7 | 31.8 | 4 | 10 |
| Normal | 4 | 22.2 | 14 | 63.6 | 36 | 90 |
| Total | 18 | 100 | 22 | 100 | 40 | 100 |
#OH: Overt hypothyroidism; *SCH: Subclinical hypothyroidism; PE: Pre-eclampsia
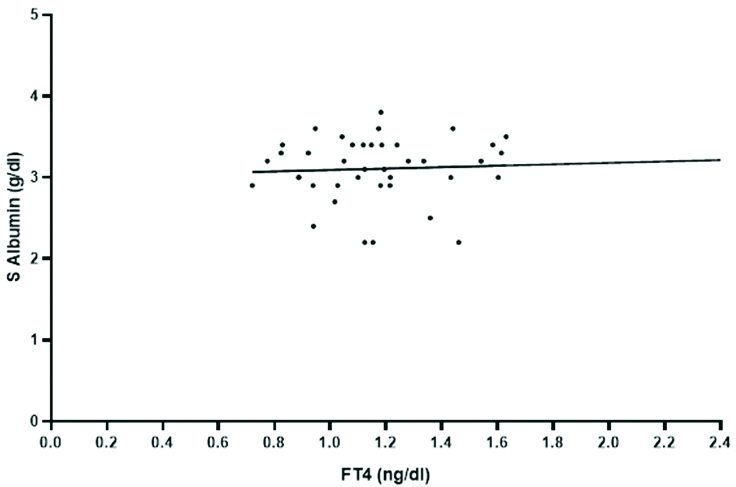
Correlation of serum albumin with thyroid profile was tested by pearsons correlation coefficient. There is negative correlation of TSH with albumin (r=-0.163). Positive correlation was seen for fT4 with albumin [Table/Fig-4]. The rest of the hormones had a negative correlation with serum albumin. There was no statistical significance for the correlation between thyroid hormones and serum albumin among the 40 cases (p>0.05).
Positive correlation between fT4 and albumin.
Chi-square test was done and odds ratio calculated for association between hypoalbuminemia and hypothyroidism in cases. Out of 22 cases with hypothyroidism, 20 (90.9%) had hypoalbuminemia. Only two cases (9.1%) of hypothyroidism had normal albumin. A 77.8% of 18 cases with normal TSH also had hypoalbuminemia [Table/Fig-5]. This was statistically not significant (p=0.247) and odds ratio was 2.85. With 95% confidence, the true odds ratio lies in the range of 0.45 to 17.81.
Distribution of hypothyroidism in all 40 cases with hypoalbuminemia.
| Hypoalbuminemia (S.Albumin <3.5 g/dL) | Hypothyroidism (TSH >3.5 mIU/L) | Total | χ2 | df | p | OR | 95% CI |
|---|
| Yes | No |
|---|
| Yes | 20 (90.9%) | 14 (77.8%) | 34 | 1.33 | 1 | 0.247 | 2.85 | 0.45 | 17.81 |
| No | 2 (9.1%) | 4 (22.2%) | 6 |
| Total | 22 (100%) | 18 (100%) | 40 | | | | | | |
Out of 14 severe pre-eclampsia patients with hypothyroidism, 12 (85.7%) had hypoalbuminemia. A 75% of normothyroid cases also had hypoalbuminemia. This was statistically not significant (p=0.6). Chi-square test was done and odds ratio was 2.0 [Table/Fig-6].
Distribution of hypothyroidism in 18 severe pre-eclampsia cases with hypoalbuminemia.
| Hypoalbuminemia (among severe pre-eclampsia) | Hypothyroidism | Total | χ2 | df | p | OR | 95% CI |
|---|
| Yes | No |
|---|
| Yes | 12 (85.7%) | 3 (75%) | 15 | 0.257 | 1 | 0.612 | 2.0 | 0.13 | 30.16 |
| No | 2 (14.3%) | 1 (25%) | 3 |
| Total | 14 (100%) | 4 (100%) | 18 | | | | | | |
All the mild pre-eclampsia cases with hypothyroidism had low albumin. An 11 (78.6%) of the normothyroid also had hypoalbuminemia. This was statistically not significant (p=0.159). Odds ratio could not be determined for mild cases as none of the cases with hypothyroidism had normal albumin [Table/Fig-7].
Distribution of hypothyroidism in 22 mild pre-eclampsia cases with hypoalbuminemia.
| Hypoalbuminemia (among mild pre-eclampsia) | Hypothyroidism | Total | χ2 | df | p | OR | 95% CI |
|---|
| Yes | No |
|---|
| Yes | 8 (100%) | 11 (78.6%) | 19 | 1.99 | 1 | 0.159 | - | - | - |
| No | 0 | 3 (21.4%) | 3 |
| Total | 8 (100%) | 14 (100%) | 22 | | | | | | |
Discussion
Pre-eclampsia is a multifactorial disease. Many studies have mentioned the role of antiangiogenic factors in pre-eclampsia [2,3,8,17]. Antiangiogenic factors like sFlt-1 (soluble forms like tyrosine kinase-1) or sVEGFR-1 (soluble Vascular Endothelial Growth Factor Receptor-1) are released by placental hypoxia. This antagonises normal functioning of VEGF [2,8]. This causes fenestration in the glomerular capillaries leading to proteinuria. These antiangiogenic factors may also affect thyroid capillaries causing high TSH [3]. Li LZ et al., suggested that proteinuria may lead to mucous membrane oedema of the gastrointestinal tract, which in turn may decrease protein intake, causing decrease in the synthetic materials required for thyroid hormone synthesis, thus reducing thyroid hormone production [6]. Lo JC et al., suggests that 10% of thyroid hormones are bound to serum albumin, 75% bound to thyroxine-binding globulin, 15% to transthyretin, and 0.03% is free [17]. Thus, proteinuria may lead to loss of thyroid hormones. In view of this hypothesis, we tested the serum albumin levels and thyroid levels in cases and controls.
Cases and controls were comparable with respect to their age, gestational age and parity. Pre-eclampsia group had significantly lower levels of albuminthan normal pregnant ladies. There was significantly higher percentage of pre-eclampsia cases with albumin below expected levels. Gojnic M et al., and Margarson MP and Soni N found low albumin in pre-eclampsia and noted that it was an indicator of its severity [4,18]. Significantly low albumin levels were also detected by McCartney CP et al., [19]. However, studies like that of Salako BL et al., has reported an increase in mean serum albumin of patients who later developed pre-eclampsia [20].
Thyroid hormone (T3, fT3, fT4) levels in cases were significantly lower in pre-eclampsia cases than in controls. TSH was found to be significantly higher in cases. Thus, in this study, a state of hypothyroidism were seen among pre-eclampsia patients. Basbug M et al., Lao TT et al., and Tolino A et al., have found a similar decrease in T3 and T4 [7,21,22]. Lao TT et al., also found higher TSH concentrations in pre-eclampsia compared to normotensive pregnancies [21]. Basbug M et al., has also noted a significant decrease in free thyroid hormones, similar to this study [7]. Kumar A et al., did not find a significant decrease in thyroid hormones, however TSH was seen to be increased in preeclamsia in their study [8]. Khadem N et al., also did not find any significant difference in either total or free thyroid hormones or TSH between pre-eclampsia patients and normal pregnant women [23].
This study also tested the correlation between serum albumin and thyroid profile. It was found that there was a negative correlation between TSH and serum albumin. Thus, as albumin decreases, TSH is seen to be raised. Li LZ et al., have noted that albumin is lower in nephritic syndrome patients with thyroid dysfunction. They concluded that proteinuria as a reason for hypothyroidism [6]. Proteinuria in pre-eclampsia leads to hypoalbuminemia. The loss of albumin could be a cause for the low total T3 and T4 levels. This results in the high TSH levels seen in pre-eclampsia in this study. However, this was statistically not significant. T3 and T4 had a negative correlation with albumin in the present study. However, Sardana D et al., observed that serum albumin levels showed a positive correlation with both T3 and T4 levels and a negative correlation with TSH levels in pre-eclampsia [24]. Lao TT et al., and Tolino A et al., also noted similar findings [21,22]. The decrease in fT4 among cases correlated positively with reduced albumin levels in the present study. Kumar A et al., found that fT4 concentration was not related to plasma albumin [8]. This could be due to lack of synthetic materials for thyroid hormone synthesis caused by gastrointestinal mucosal changes associated with proteinuria [6]. However, low fT3 levels showed a negative correlation with low albumin in present study. Lao TT et al., observed that the thyroid hormones, with the exception of fT4 were correlated with albumin [21].
Hypoalbuminemia was seen in 90.9% of the pre-eclampsia cases with hypothyroidism in the present study. Although, the odds of pre-eclampsia patients with hypoalbuminemia developing hypothyroidism was 2.85 times more, compared to patients without hypoalbuminemia but it was not statistically significant. The odds of severe pre-eclampsia group with hypoalbuminemia developing hypothyroidism were two times more than severe pre-eclampsia with normal albumin. There were no mild pre-eclampsia cases with hypothyroidism.
Limitation(s)
This study does not follow-up the patients to see if hypothyroidism resolves after albumin levels or proteinuria return to normal following management of pre-eclampsia or postdelivery. Also, other causes of hypothyroidism like antiangiogenic factors, or thyroid autoantibodies have not been tested. Future studies that test the association between antiangiogenic factors and hypothyroidism could be done. Further studies with larger sample size may be necessary to find the risk factors associated with hypothyroidism in pre-eclampsia, which could also help in the prevention or management of hypothyroidism.
Conclusion(s)
In this study, there is significant hypoalbuminemia in pre-eclampsia patients as compared to normal pregnant ladies in the same trimester. The risk of hypothyroidism in pre-eclampsia patients with hypoalbuminemia was 2.85 times compared to those with normal albumin levels. In severe pre-eclampsia, this risk was seen to be two times, while none of the mild pre-eclampsia patients with low albumin had hypothyroidism. In our study there was a state of hypothyroidism where high TSH and low fT4 correlated with low albumin levels, however this was not statistically significant. Thus hypoalbuminemia is not a risk factor for the hypothyroidism in pre-eclampsia patients, according to this study.
#OH: Overt hypothyroidism; *SCH: Subclinical hypothyroidism; PE: Pre-eclampsia