Tubercular Empyema Thoracic: An Acute Presentation with COVID-19 Co-infection
Sachin Gautam1, Sanjay Pandit2, Dharam Pal Bhadoria3, Rahul Kumar4
1 Postgraduate Student, Department of Medicine, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi, India.
2 Professor, Department of Medicine, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi, India.
3 Director Professor, Department of Medicine, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi, India.
4 Postgraduate Student, Department of Medicine, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sachin Gautam, D-11, S2, 2nd Floor, Dilshad Colony, Delhi-110095, India.
E-mail: sachingautam26273@gmail.com
COVID-19 (Coronavirus Disease 2019) pandemic is caused by a novel Coronavirus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), which has received worldwide attention and most COVID-19 patients have respiratory symptoms and develop a mild disease. In addition, co-infection of SARS-CoV-2 with other respiratory infections of bacterial, other viral and fungal origin needs to be validated. The clinical features, course and treatment of tuberculosis (TB) patients with COVID-19 are unclear and understudied. There is paucity of literature on this co-infection. Here, authors present a case report of a 35-year-old diabetic Asian male patient who presented to the emergency department as COVID-19 positive with acute exacerbation of symptoms, who later developed respiratory distress and was eventually found to have a lung abscess with subsequent tubercular empyema thoracic on contrary to a severe COVID-19 pneumonia or a fungal infection. Contrast Enhanced Computed Tomography (CECT) chest along with Cartridge-Based Nuclei Acid Amplification Test (CBNAAT) of pleural fluid pus confirmed the tubercular lesion timely to aid the diagnosis and further course of management.
Coronavirus, Lung abscess, Mycobacterium tuberculosis
Case Report
A 35-year-old COVID-19 positive male patient presented to the COVID emergency with a history of recent onset of fever associated with non-productive cough since 4 to 5 days and shortness of breath since two days. Patient had no history of weight loss, loss of appetite, contact with a TB patient in neighborhood or previous hospitalisation. Family history was insignificant. Patient was on mixtard human insulin (30/70) regimen as 20 units subcutaneously before breakfast and 10 units subcutaneously before dinner for treatment of diabetes since two years, on which diabetes was apparently controlled. He smoked one bundle of bidi daily and consumed 28 g of hard liquor daily since 6-7 years. On examination patient was conscious and oriented, febrile (38°C), blood pressure of 80/50 mmHg, pulse rate of 106 per minute, respiratory rate of 27 per minute, oxygen saturation of 93% under room air. Chest auscultation revealed bilateral crepitations (right >left) along with reduced air entry on left-side and bronchial breath sounds over left supraclavicular region. Rest of the general physical and systemic examination was unremarkable.
A random blood glucose of 485 mg/dL with urine for ketones one positivity along with normal arterial blood gas was noted. Remarkable laboratory test findings on the day of admission have been tabulated in [Table/Fig-1]. Rest of the laboratory investigations were within normal limits. Chest radiograph on the day of admission was suggestive of left-sided pleural effusion with bilateral lower respiratory tract infection as shown in [Table/Fig-2]. Patient’s Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) nasal and throat swab for Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS CoV-2) was also performed inside the lab. A working diagnosis of COVID-19 positive with diabetic ketosis with severe acute respiratory infection with sepsis with septic shock was provisionally made. Urine and blood samples were collected before starting the antibiotics.
Relevant laboratory findings.
| Serial No. | Blood parameters, on the day of admission with normal range | Values |
|---|
| 1. | Total leukocyte count (5000-10,000 cells/ mm3) | 14530 cells/mm3 |
| 2. | Differential leukocyte count (60-75% polymorphs, 20-40% lymphocytes, monocytes 2-6%) | 92% neutrophils, 16% lymphocytes, and 2% monocytes |
| 3. | Erythrocyte sedimentation rate (By Westergren: 0-9 in males) | 48 |
| 4. | Serum lactate dehydrogenase (125-246 U/L) | 882 U/L |
| 5. | Highly sensitive C reactive protein (<5 mg/L) | 90 mg/L |
| 6. | Procalcitonin (0.0-0.5 ng/mL) | 0.95 ng/mL |
| 7. | Serum ferritin level (30-400 ng/mL in males) | 369 ng/mL |
| 8. | Interleukin-6 level (<7 pg/mL) | 22 pg/mL |
| 9. | D-dimer of (<500 ng/mL) | 1112 ng/mL |
| 10. | International normalised ratio (0.8 to 1.2) | 0.90 |
| 11. | HbA1c (Glycated Haemoglobin) | 11.5% |
| 12. | On day fourth-Blood, Urine and Pus culture for bacterial and fungal elements. | Sterile after 48 hours of incubation |
| 13. | HIV, Hepatitis B and Hepatitis C serology | Negative |
Chest radiograph on presentation- suggestive of inhomogeneous opacities in bilateral lung fields with left-sided pleural effusion.
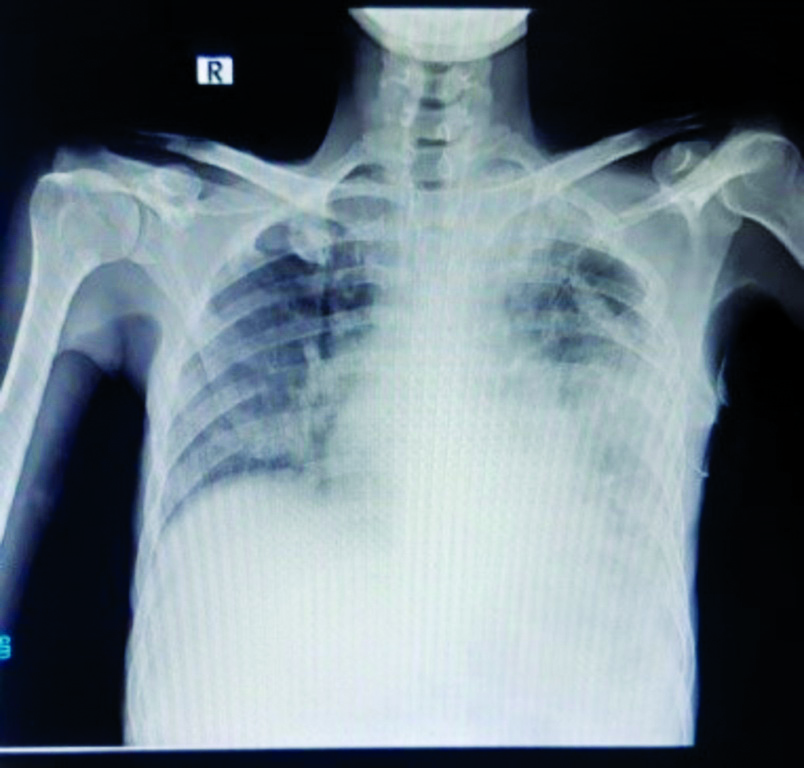
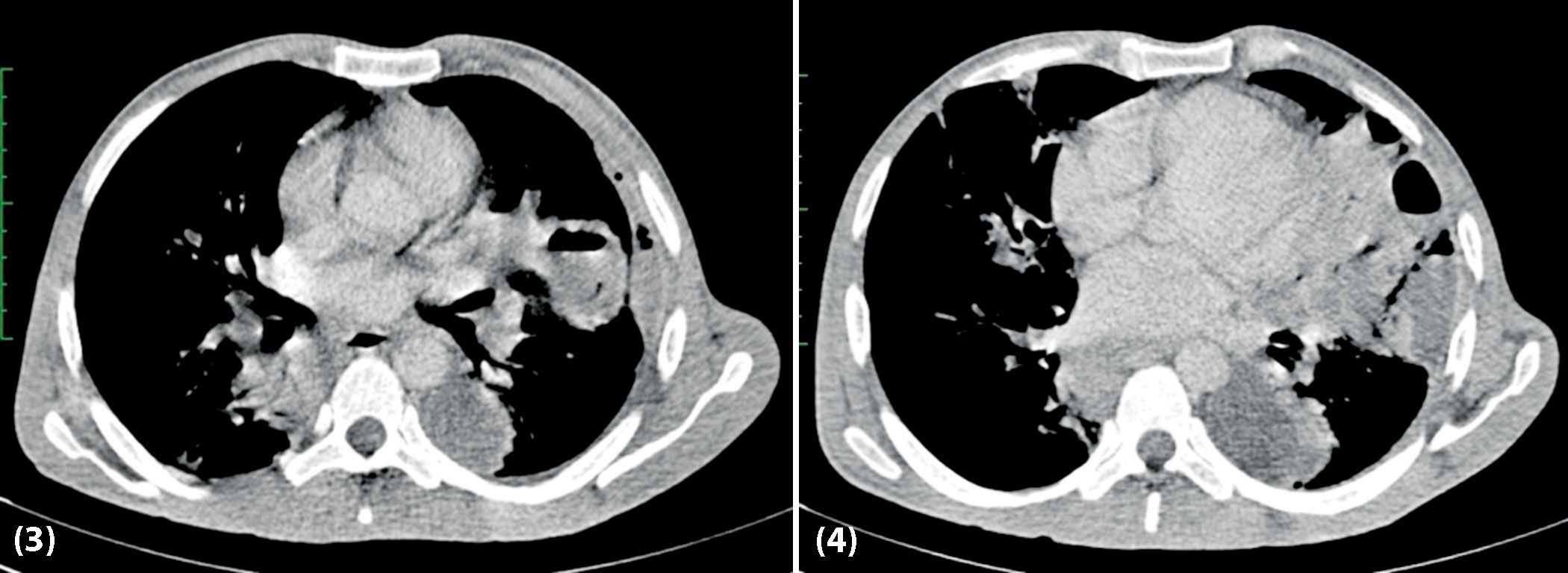
Treatment began with oxygen support via venturi mask at 10 L/min, noradrenaline and plain insulin infusion injection was started, which was later replaced by a dextrose insulin drip. Injection piperacillin and tazobactam along with clindamycin and fluconazole injection were started. On second day, as blood pressure improved, inotrope support was stopped, however respiratory distress worsened, and oxygen saturation started falling below 91%. Patient’s RT-PCR swab reported positive for SARS-COV-2. Prophylactically methyprednisolone and enoxaparin injection were started. A CECT chest was performed on second day [Table/Fig-3,4 and 5], which reported a lung abscess in left upper lobe with pyopneumothorax, following which injection vancomycin was added to the treatment regimen. An intercostal chest drain was inserted in left fifth intercostal space with a resultant reduction in work of breathing and a frank pus of 200 mL was drained initially.
CECT chest (mediastinal window): Shows evidence of a well-defined cavity of 8 mm thickness in left upper lobe, with air fluid level. Also, presence of left-sided loculated pleural effusion of 2×3.5 cm in dimension with pyo-pneumothorax.
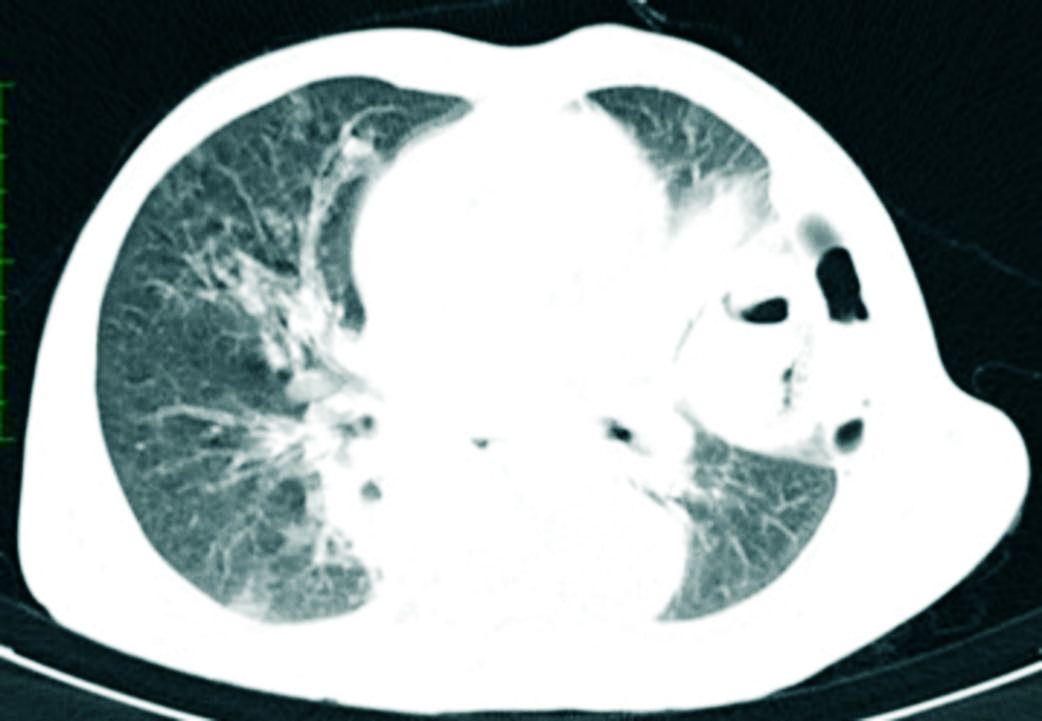
CECT chest (lung window): Fibro atelectatic opacities in left lower lobe and ground glass nodules in right upper lobe with areas of consolidation in right lower lobe, findings suggestive of infective etiology likely bacterial.
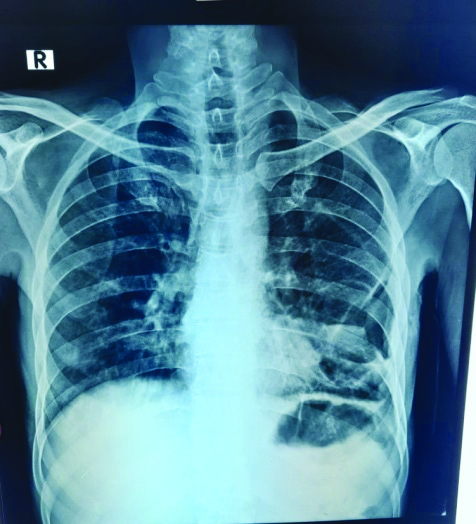
Pus culture for bacterial and fungal growth was collected and pus analysis for CBNAAT detected Mycobacterium tuberculosis without rifampicin resistance. Patient’s weight was recorded as 43 kilograms. The working diagnosis was now changed to COVID-19 positive with diabetic ketosis with sepsis with left upper lobe tubercular lung abscess and left-sided tubercular empyema thoracis with severe acute respiratory infection on third day of admission. Injection methylprednisolone was stopped, weight based antitubercular drugs were started (isoniazide 200 mg/day, rifampicin 450 mg/day, pyrazinamide1000 mg/day and ethambutol 800 mg/day) along with tablet pyridoxine 20 mg once a day. As patient started accepting orally, a high protein and diabetic diet along with a fixed basal bolus insulin regimen were started. Patient’s blood, urine and pus culture for bacterial and fungal growth reported sterile after 48 hours of incubation on fourth day, [Table/Fig-1]. On sixth day, patient’s intercostal chest tube was removed following which oxygen support, injection fluconazole and prophylactic enoxaparin were stopped. From seventh day onwards patient’s fever subsided, total leukocyte count started coming within normal range and antibiotics were stopped after 10 days. Finally, patient was discharged with home quarantine for 10 days after being reported negative for SARS-CoV-2 on 12th day of admission [Table/Fig-6].
Post Intercostal Drainage Tube (ICD) removal, chest radiograph on day 12th of admission shows resolution of earlier radiological findings along with lung expansion on left-side.
Post-discharge patient reported improvement in general health, appetite and well being on telephonic consultation. Patient was advised to follow-up in a non-COVID-19 medicine OPD with regular monitoring of haemogram, liver and kidney function tests. Written informed consent was obtained from the patient for this study.
Discussion
Ever since the declaration of COVID-19 disease as pandemic by World Health Organisation (WHO), the paradigm of medical research has been shifted towards SARS-CoV-2 [1]. A study by Oei W and Nishiura H in 2012, estimated a higher mortality among patients of influeza co-infected with TB [2]. Studies are available where co-infection of SARS-CoV-2 with other respiratory pathogens like influenza virus as well as tuberculosis with other coronaviruses like SARS-CoV-1 and Middle East Respiratory Syndrome- Corona Virus (MERS-CoV) have been mentioned [3-6]. However, there is paucity of literature on co-infection of SARS-CoV-2 with a cavitary TB lung disease. Recent reviews on TB and COVID-19 co-infection cohorts have been tabulated in [Table/Fig-7] [7-12].
Review of recent TB/COVID-19 cohorts published so far.
| Serial No. | Reference | Country | Sample size (Co-infected/Total) | Clinical features | Outcomes (% Deaths) | Study main findings | Limitation(s) |
|---|
| 1. | Tadolini M et al., [7] | Multinational | 49/49 | Mainly males, 43 were symptomatic. 36 with active TB and another 13 with a previous history of TB. Other co-morbidities were also present in some like- HIV infection, diabetes and malignancy | 18 patients recovered, 25 patients were still on treatment and 6 patients expired | Role of SARS-CoV-2 in the progression from latent TB infection to the active disease, as well as the role of Mycobacterium tuberculosis in the progression of COVID-19, requires larger studies. It was also found COVID-19 also occurred in seven patients with post TB sequelae. These subjects had higher mortality and were older to other subjects. (Although not statistically significant) | Being it multi-national, cohorts were heterogeneous, with differences in therapeutic protocols and access to health care services. Hence not to be considered a representative of Global or European perspective. |
| 2. | Chen Y et al., [8] | China | 36/86 | Co-infected subjects quickly developed respiratory symptoms, and had severe clinical manifestation | Not available | It was found that TB infections increases the susceptibility to SARS-CoV-2 infections and its severity | The inclusion criteria on classifying subjects with TB was not very specific, hence cases had heterogeneous clinical manifestations. There was also lack of information on co-morbidities among the subjects and also on social determinants. |
| 3. | Stochino C et al., [9] | Italy | 20/20 | Majority were males, 13 subjects had lymphocytopenia and one had thrombocytopenia. one patient expired which had severe respiratory failure. Biochemical tests like D-dimer levels showed deviations from expected values, rest did not | 12 patients recovered, five patients were still on treatment and one patient expired | In a highly vulnerable population, TB/COVID-19 co-infection is likely manageable with proper care, rigorous infection control practices and personal protection devices to prevent the risk of in-hospital transmission further | As most of the subjects were recent immigrants, clinical symptoms were not assessed properly due to cultural and linguistic barriers as well as long-term outcomes as follow-up was limited to few weeks only. |
| 4. | Motta I et al., [10] | Italy | 69/69 | Co-morbidities such as hypertension, alcoholism and diabetes were prevalent among patients who expired and most of them were elderly males | 61 patients recovered and 8 patients expired | Elderly patients with co-morbidities; have a higher risk of mortality; TB might not be a major determinant of mortality; Migrants had lower mortality as most of them were of younger age with lower number of co-morbidities | Cohorts were heterogeneous, with differences in therapeutic protocols and access to health care services. Hence not to be considered a representative of Global or European perspective. |
| 5. | Sy KTL et al., [11] | Philippines | 172/860 | Majority of patients were males, most of them with co-morbidities like hypertension and/or diabetes. Elderly and individuals with multiple co-morbidities had most of the mortality. | 95 patients recovered and 43 patients expired. (34 unknown) | In COVID-19 patients, co-infection with TB, increased the morbidity and mortality | Study had limited information on social determinants and co-morbidities that may be influencing the co-infection and prognosis. |
| 6. | Davies MA [12] | South Africa | 2128/22308 | Majority of patients were females, most of them with co-morbidities like hypertension and/or diabetes. Elderly and individuals with multiple co-morbidities had most of the mortality. | Co-infected HIV seronegative individuals 879 Previous TB + COVID-19: 45 deaths 155 Current TB + COVID-19: 10 deaths Co-infected HIV seropositive individuals 864 Previous TB + COVID-19: 42 deaths 172 Current TB + COVID-19: 16 deaths | In patients infected by COVID-19, it was found that both past history of TB, current TB and TB associated with HIV increases the risk of mortality among them | Study had limited information on social determinants and co-morbidities that may be influencing the co-infection and prognosis. |
The precise mechanism governing COVID-19 and TB co-infection is still not known, however with a transient decrease in cellular immunity both could augment each other, as a new infection or exaggerated reactivation of latent infection, role of various interleukins that affect T-cell immune response in response to viral inflammation is an argument mentioned in the literature [13]. Inappropriate activation of type-I interferon, having antiviral properties, in viral infections like COVID-19 may promote susceptibility to TB infection [4]. Respiratory symptoms like fever, cough, breathlessness, and weakness, are present in both TB and SARS-CoV-2 infection, hence the differentiation between the two is quite tough on clinical ground, however there is slow progression of symptoms in TB as compared to rapid progression in case of COVID-19. But in this case, patient had no history of previously treated TB, constitutional chronic symptoms like loss of weight or loss of appetite, however being a resident in endemic region for TB made him a high risk subject. A systematic review by Al-Rifai RH et al., stated that diabetes mellitus increases the risk of developing active TB infection by two to four times, furthermore a study conducted by Guo W et al., found that dysregulated glucose metabolism is itself a higher risk factor for developing COVID-19 pneumonia and its rapid progression [14,15]. This patient initially developed diabetic ketosis which later developed into sepsis and to resolve it intense glucose control measures and monitoring was done. In this case report radiological findings of chest radiograph were inconsistent for COVID-19 and for typical tubercular cavitary lung lesion, however, CECT chest along with CBNAAT of pleural fluid pus helped in making the final diagnosis. CBNAAT for Mycobacterium tuberculosis has yielded variable results with sensitivity ranging between 42 to 100% and specificities ranging between 85 to 100% [16]. Secondary fungal infections have also been reported in COVID-19 patients as evidenced from a study in Wuhan, China which reported 3 out of 9 (33.3%) patients and 6 out of 17 (35.3%) critically ill patients developing secondary fungal infection [17]. However, this patient did not develop a fungal infection. From this case, it is evident that radiological findings of COVID-19 could be overlooked in a case of co-infection of COVID-19 with a cavitary tubercular lung disease. Also, it was found in a study from Italy that no characteristic radiological features for COVID-19 are found in most of the cases with co-infection [9]. Lung cavitation due to COVID-19 pneumonia is also uncommon [18]. The purpose of reporting this case was to highlight the importance of considering the possibility of co-existing infections like TB in a COVID-19 positive patient, who experienced a sudden worsening of respiratory distress on contrary to severe COVID-19 infection, as thought of, especially in India. A case report by Yousaf Z et al., included six patients with SARS-CoV-2 and TB co-infection, which highlighted the importance of detecting this co-infection earlier so as to avoid unnecessary community spread and timely management of COVID-19 [19]. It is still unclear whether COVID-19 flares up a latent tubercular infection or slows down its own progression in a co-infection. A systematic review and meta-analysis performed by Gao Y et al., revealed patients with TB are less likely to get COVID-19, but serious complications from COVID-19 are more likely to develop in patients with pre-existing tuberculosis [20]. Although this patient did not develop severe COVID-19 pneumonia, but the role of steroids and immunosuppressive drugs like IL-6 inhibitor (Tocilizumab) for COVID-19 requires more studies and literature especially in TB and COVID-19 co-infected patients.
Conclusion(s)
In the ongoing pandemic of COVID-19, clinicians should suspect the possibility of COVID-19 and TB co-infection, especially in India. Early recognition of this co-infection can help to prioritise the therapeutic strategies and prevent transmission in community as both diseases encompass the possibility of altering the clinical presentation, course, management and outcome of the patient.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jan 07, 2021
Manual Googling: Apr 10, 2021
iThenticate Software: May 13, 2021 (16%)
[1]. World Health Organisation (WHO). Coronavirus disease 2019 (COVID-19) situation report – 51. 2020. March 11 Accessed 2020 Mar 24. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 [Google Scholar]
[2]. Oei W, Nishiura H, The relationship between tuberculosis and influenza death during the influenza (H1N1) pandemic from 1918-19Comput Math Methods Med 2012 2012:12486110.1155/2012/12486122848231 [Google Scholar] [CrossRef] [PubMed]
[3]. Ding Q, Lu P, Fan Y, Xia Y, Liu M, The clinical characteristics of pneumonia patients co-infected with 2019 novel coronavirus and influenza virus in Wuhan, ChinaJ Med Virol 2020 92:1549-55.10.1002/jmv.2578132196707 [Google Scholar] [CrossRef] [PubMed]
[4]. Donovan ML, Schultz TE, Duke TJ, Blumenthal A, Type I interferons in the pathogenesis of tuberculosis: Molecular drivers and immunological consequencesFront Immunol 2017 8:163310.3389/fimmu.2017.01633229230217 [Google Scholar] [CrossRef] [PubMed]
[5]. Liu W, Fontanet A, Zhang PH, Zhan L, Xin ZT, Tang F, Pulmonary tuberculosis and SARS, ChinaEmerg Infect Dis 2006 12:707-09.10.3201/eid1204.0502643294680 [Google Scholar] [CrossRef] [PubMed]
[6]. Alfaraj SH, Al Tawfiq JA, Altuwaijri TA, Memish ZA, Middle East respiratory syndrome coronavirus and pulmonary tuberculosis co-infection: Implications for infection controlIntervirology 2017 60:53-55.10.1159/00047790828683463 [Google Scholar] [CrossRef] [PubMed]
[7]. Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar JW, Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 casesEur Respir J 2020 56(1):200139810.1183/13993003.02328-202032586888 [Google Scholar] [CrossRef] [PubMed]
[8]. Chen Y, Wang Y, Fleming J, Yu Y, Active or latent tuberculosis increases susceptibility to COVID-19 and disease severityMedRxiv 2020 202010.1101/2020.03.10.20033795 [Google Scholar] [CrossRef]
[9]. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC, Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospitalEur Respir J 2020 56:200170810.1183/13993003.01708-202032482787 [Google Scholar] [CrossRef] [PubMed]
[10]. Motta I, Centis R, D’Ambrosio L, García-García JM, Goletti D, Gualano G, Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohortsJ Pulmonol 2020 26:233-40.10.1016/j.pulmoe.2020.05.00232411943 [Google Scholar] [CrossRef] [PubMed]
[11]. Sy KTL, Haw NJL, Uy J, Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19Infect Dis (Auckl) 2020 52(12):902-07.10.1101/2020.07.22.20154575 [Google Scholar] [CrossRef]
[12]. Davies MA, HIV and risk of COVID-19 death: A population cohort study from the western cape province, South Africa. Author: western cape department of health in collaboration with the national institute for communicable diseases, South Africa. Corresponding autMedRxiv 2020 :01-21.10.1101/2020.07.02.20145185 [Google Scholar] [CrossRef]
[13]. Redford PS, Mayer-Barber KD, McNab FW, Stavropoulos E, Wack A, Sher A, Influenza a virus impairs control of Mycobacterium tuberculosis Co-infection through a type I interferon receptor–Dependent pathwayJ Infect Dis 2014 209:270-74.10.1093/infdis/jit42423935205 [Google Scholar] [CrossRef] [PubMed]
[14]. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ, Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysisPLoS One 2017 12:e018796710.1371/journal.pone.018796729161276 [Google Scholar] [CrossRef] [PubMed]
[15]. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Diabetes is a risk factor for the progression and prognosis of COVID-19Diabetes Metab Res Rev 2020 36:e331910.1002/dmrr.33197228407 [Google Scholar] [CrossRef] [PubMed]
[16]. Sinzobahamvya N, Bhakta HP, Pleural exudate in a tropical hospitalEur Respir J 1989 2:145-48. [Google Scholar]
[17]. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019Clin Infect Dis 2020 71(8):1937-42.10.1093/cid/ciaa44932301997 [Google Scholar] [CrossRef] [PubMed]
[18]. Chen Y, Chen W, Zhou J, Sun C, Lei Y, Large pulmonary cavity in COVID-19 cured patient case reportAnn Palliat Med 2020 9:05-452.10.21037/apm-20-45232527136 [Google Scholar] [CrossRef] [PubMed]
[19]. Yousaf Z, Khan AA, Chaudhary HA, Mushtaq K, Parengal J, Aboukamar M, Cavitary pulmonary tuberculosis with COVID-19 co-infectionIDCases 2020 22:e0097310.1016/j.idcr.2020.e0097333014710 [Google Scholar] [CrossRef] [PubMed]
[20]. Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J, Association between tuberculosis and COVID-19 severity and mortality: A rapid systematic review and meta-analysisJ Med Virol 2021 93:194-96.10.1002/jmv.2631132687228 [Google Scholar] [CrossRef] [PubMed]