Haematological diseases are disorders that primarily affect the blood and blood-forming organs. Haematological malignancies include a number of cancer types that originate from bone marrow or lymphatic system blood cells and are among the top 10 malignancies in terms of frequency and cause of death in cancer patients [1]. Cytogenetic analysis has become a standard procedure in the initial phase of diagnosis in many types of haematological diseases, and is an important aid in the classification of these disorders [2]. Recurrent chromosomal abnormalities are involved in the pathogenesis of haematological malignancies and are important indicators for their diagnosis and prognosis. The value of conventional cytogenetics lies in its complete overview of the genome, and banding plays a key role in the detection of each of the 46 chromosomes and their structural and numerical changes [3].
The aim of this study was to evaluate the role of cytogenetic methods in the diagnosis of haematological diseases. The hypothesis states that cytogenetic methods will show a significant role in the detection and diagnosis of haematological diseases.
Materials and Methods
The present study was a retrospective cohort analysis of karyograms and findings of patients with various haematological diseases, made over a period of three consecutive years i.e., from 2016 to 2019 and analysis of data was done from April to June 2020. Peripheral blood samples were used for the cytogenetic diagnosis of haematological diseases. Cultivation of samples and their preparation for analysis was done using a standardised protocol with certain modifications specific to the laboratory [4]. Karyotyping was performed according to the recommendations of the ISCN (International System of Human Cytogenetic Nomenclature) [5]. Complete cytogenetic processing of samples and their evaluation was done at the Centre for Genetics at the Medical Faculty of the University of Sarajevo in Bosnia and Herzegovina.
The inclusion and exclusion criteria for participating in this study were a definitive diagnosis of a haematological malignancy and a detailed cytogenetic finding. Thus, 69 patients were excluded from the further analysis: 33 due to inadequate diagnosis (disease other than haematological malignancy); 21 due to incomplete cytogenetic finding; 12 due to the lack of final diagnosis; two had repeated findings, and for one person the cytogenetic analysis was unsuccessful due to a lack of material.
In total, there were 215 patients who underwent analyses with suspected haematological disease at the Centre for Genetics during the aforementioned three years.
The findings of the remaining 146 patients (75 females and 71 males) were evaluated in this research. The present study was performed in accordance with the ethical standards and the Declaration of Helsinki.
Statistical Analysis
After the conducted research, the obtained data were grouped, analysed using Microsoft Excel 2019 and presented in tables and graphs.
Results
A total of seven different diagnoses of haematological diseases were determined, where Chronic Myeloid Leukaemia (CML) was the most common. Other diagnoses and their frequency are presented in [Table/Fig-1].
Diagnoses and their frequency.
| Diagnosis | Total number of patients | Frequency of diagnosis |
|---|
| Chronic Myeloid Leukaemia (CML) | 45 | 30.82% |
| Acute Lymphoblastic Leukaemia (ALL) | 24 | 16.44% |
| Acute Myeloid Leukaemia (AML) | 24 | 16.44% |
| Non-Hodgkin’s Lymphoma (NHL) | 16 | 10.96% |
| Myelodysplastic Syndrome (MDS) | 13 | 8.90% |
| Chronic Lymphocytic Leukaemia (CLL) | 13 | 8.90% |
| Myeloproliferative Disorders (MPD) | 11 | 7.53% |
The mean age of all patients at the time of diagnosis was 51.5 years. Patients were divided into one of the eight age groups (<20, 21-30, 31-40, 41-50, 51-60, 61-70, 71-80 and >80 years old). Most patients were in the age group of 61-70 years (21.53%), and the least were in the group of over 80 years of age (2.08%).
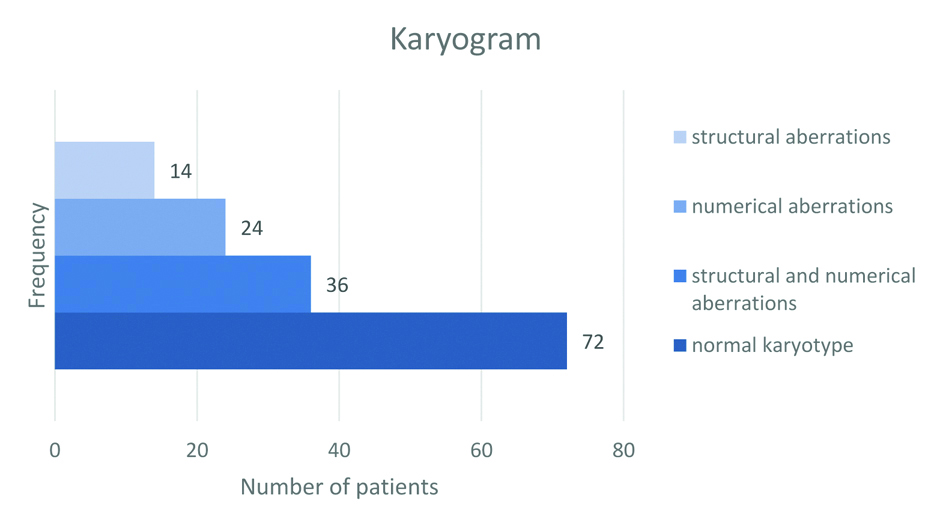
As part of cytogenetic findings, based on cytogenetic tests of lymphocyte culture, the number and morphology of chromosomes were analysed according to the standard procedure for each patient. The karyogram was normal in 49.32% (72) of patients, while chromosomal abnormalities were observed in the remaining 50.68% (74) of patients. The frequency of observed aberrations or of their absence is graphically shown in [Table/Fig-2].
Frequency of observed aberrations.
Consistent with the fact that CML was the most common diagnosis, it was found in 19 males and 26 females, making it the most common disease in both sexes. 44.44% (20) of CML patients had a normal karyogram, while chromosomal abnormalities were observed in 55.56% (25) of patients. Chromosomal aberrations that were manifested in CML patients and were not previously described were: lack of chromosomes (11, 13, 19 and 22), additional copies of chromosomes (9, 18, and X), trisomy (7, 9, 11, 12), lack of p arms (4, 19), lack of q arms (6, 15, 21), translocation t(14q;18p), inversions inv 9 (p11q12) and inv 11, and isochromosomes 11 and 16.
The largest number of Acute Lymphoblastic Leukaemia (ALL) patients, i.e. 54.17% (13) had a normal cytogenetic finding and 45.83% (11) of patients had manifested chromosomal aberrations. Certain abnormalities that are not common for ALL were found: trisomy 18 and 20, monosomy 4, lack of chromosomes X, Y and 22, isochromosomes 11, 16 and 17, translocations t(7;12)(q33,q24), t(6,3)(p-,q+), and tumour markers type D and G.
As for the Acute Myeloid Leukaemia (AML) patients, a normal cytogenetic finding was found in 29.17% (7) of them. Patients diagnosed with AML most often had numerical aberrations, i.e. 33.33% (8) of them, structural anomalies were observed in 12.5% (3) of subjects, and both structural and numerical changes were observed in 25% (6) of cases. Well-known aberrations were found, such as: del 7/7q, del 5/5q, trisomy 8, t(15;17)(q22;q21) and t(8;21)(q22;q22.1). In addition to that, some less common structural aberrations were observed in the patient sample: t(8;13)(p12;q12), t(1p;10p), t(7;9)(q33-36 or q34-36;q34), t(7;14), t(2;5), t(6;21)(p21;q22) and t(4;8)(q28;q24).
As part of this study, 43.75% (7) of patients diagnosed with Non-Hodgkin's lymphoma (NHL) had a normal cytogenetic finding. Chromosomal aberrations were observed in the remaining 56.25% (9) of patients, of whom 88.9% (8) had complex karyotypes. Some of the characteristic chromosomal aberrations associated with certain NHL subtypes were found: translocations t(14;18)(q32;q21), t(8;14)(q24;q32), t(3;14)(q27;q23), p arm deletions (1, 17), q arm deletions (7, 11, 13), and trisomy 18. In addition to that, some uncommon chromosomal anomalies were observed: translocations t(6q+;7q-), t(18q;14q), t(1p;2p), t(1q;3p), t(3;22)(q27;q11), t(12;21)(p12;q22), lack of p arm (2, 6, 12, 20), lack of q arm (2, 3, 14, 16, 17), additional q arm (5, 6), chromosome 3 inversion, and trisomy 6 and 13.
Out of the total number of subjects diagnosed with Myelodysplastic Syndrome (MDS), 46.15% (6) had chromosomal aberrations, of which slightly less than half (2) had a complex karyotype, which carries poor general survival. 53.85% (7) of patients had a normal cytogenetic finding. Aberrations observed in MDS patients were: translocations t(7;14), t(11;17)(q22;q34), deletion of q arm of chromosome 9, additional q arm of chromosome 1, isochromosome 16, presence of tumour markers, additional chromosomes 1, 4 and 11, and deletion of chromosome 5.
A 61.54% (8) of patients diagnosed with Chronic Lymphocytic Leukaemia (CLL) had a normal cytogenetic finding. Another 15.38% (2) of individuals had numerical aberrations, and 7.69% (1) had structural anomalies. Both structural and numerical chromosomal abnormalities were observed in the remaining 15.38% (2) of patients. Aberrations found in CLL patients were: t(6q+,12q-), t(3p-,18p+), del (7)(q22), 7q+, type C marker, hypodiploidy, and deletions of chromosomes 22 and X.
A normal karyogram was observed in 90.91% (10) of patients with Myeloproliferative Disorders (MPD), while 9.09% (1) had structural and numerical chromosomal aberrations. Observed structural anomalies included 1q- and 2p-, isochromosome 17, tumour marker type D, and translocation t(3;21)(q26;q22); while numerical abnormalities included Y chromosome deletion.
All of the chromosomal aberrations related to certain diseases that were not previously described in the literature and that were discovered during this study are represented in [Table/Fig-3].
Chromosomal aberrations uncommon for several haematological malignancies.
| Disease | Novel chromosomal aberrations |
|---|
| CML | -11, -13, -19, -22, +9, +18, +XX,trisomy (7, 9, 11, 12),4p-, 19p-, 6q-, 15q-, 21q-,t(14q;18p),inv9(p11q12), inv11,iso(11, 16) |
| ALL | trisomy (18, 20), monosomy 4,-22, -X, -Y,iso (11, 16, 17),t(7;12)(q33;q24), t(6;3)(p-,q+),tumour markers (D,G) |
| AML | t(8;13)(p12;q12), t(1p;10p),t(7;9)(q33-36 or q34-36;q34), t(7;14), t(2;5),t(6;21)(p21;q22), t(4;8)(q28;q24) |
| NHL | t(6q+;7q-), t(18q;14q), t(1p;2p), t(1q;3p),t(3;22)(q27;q11), t(12;21)(p12;q22),2p-, 6p-, 12p-, 20p-,2q-, 3q-, 14q-, 16q-, 17q-, 5q+, 6q+,inv3, trisomy (6, 13) |
| CLL | del(7)(q22), 7q+, -22, -X |
Discussion
Of the 25 CML patients with chromosomal aberrations, 48% had a manifested Philadelphia chromosome, which is significantly lower than the usual frequency found in the literature, where it is stated that the Ph chromosome is visible in approximately 90% of patients with CML [6]. Conventional cytogenetics can detect t(9;22) in most CML patients; however, an additional 2.5% of cases with sub microscopic translocations can only be observed with the use of molecular methods [7]. This drawback of cytogenetics could be responsible for the reduced frequency of Ph chromosome found in this study. Currently, the only feature associated with tyrosine kinase inhibitor treatment failure is the presence of Additional Cytogenetic Abnormalities (ACAs), which are defined as the presence of additional structural or numerical aberrations in addition to t(9;22)(q34;q11) [8]. ACAs have been observed in about 5-10% of CML patients diagnosed during CP (chronic phase) and result in CML progression from chronic phase to accelerated phase (AP) and blast crisis (BC). In this study, additional structural or numerical aberrations were found in 38% of CML patients. Within this percentage, 12% of individuals had a manifested presence of Ph chromosome, which is in line with previously published data [9].
The incidence of ACAs is higher in CML-BC than in CML-CP or AP patients. In addition, CML-BC patients have much more complex karyotypes compared to patients in other stages of this disease [10]. This information could explain the finding of additional, previously undescribed, chromosomal abnormalities, since there was no data available about the disease phase of patients assessed in this study, and most of the previously described ACAs in the literature relate to CML-CP patients. CML occurs in all age groups, but most commonly between the ages of 46 and 53 [11]. This data is consistent with the one found during this investigation. The largest number of CML patients (26.7%) belonged to the 41-50 age group, while 20% of them had between 31 and 40 years. CML is slightly more common in men than in women [12]. However, this study’s sample gives somewhat different results, with a higher prevalence in females (female: male ratio is 1.16: 0.84).
Certain abnormalities that are not common for ALL were found. However, since most ALL trials, due to the sheer frequency of this disease, have been performed in paediatric patients, there is a lack of reported genetic data for adult patients. The largest number of patients were in the age group of up to 20 years (25%), as well as in the group between 51 and 60 years (25%), which is consistent with the usual bimodal distribution of this illness. Given that ALL is relatively uncommon in young adults, no patients were recorded in the 21 to 30 age group.
In AML, cytogenetic abnormalities divide patients into three prognostic groups: favourable, moderately favourable, or unfavourable [13]. Of the 70.83% (17) of AML patients who had cytogenetic aberrations, the largest number (58.8%) belonged to the medium-risk group, and the smallest (11.8%) was in the favourable prognostic group, while the remaining (29.4%) patients had unfavourable prognostic cytogenetic abnormalities. There are at least eight different molecular subtypes of AML that are defined by repetitive genomic rearrangements and the World Health Organisation recognises them as different clinical pathological entities. Within cytogenetic findings of this study, two entities were observed, namely PML-RARA, defined by t(15;17)(q22;q21) and RUNX1-RUNX1T1, defined by t(8;21)(q22;q22.1). Age is one of the strongest risk factors for the development of AML, with ~74% of patients aged ≥55 years. In a multivariate analysis, age represents one of the most unfavourable prognostic indicators for response to treatment and general survival [14]. The largest number of AML respondents was over 50 years old (50%). A significant percentage of patients (29.2%) belonged to the 31-40 age group, while in the 21-30 group no case of AML was recorded.
Chromosomal translocations in NHL are not completely sensitive, nor specific for a particular disease subtype. Nevertheless, the detection of recurrent chromosomal abnormalities remains important in its research, especially due to its applications in disease diagnosis, classification, and prognosis [15]. Secondary genetic changes associated with mantle cell lymphoma have been observed in NHL patients, namely: deletions of 1p, 11q, 13q and 17p. Of the most common aberrations seen in follicular lymphoma, t(14;18)(q32;q21), t(8;14)(q24;q32), and trisomy 18 were observed. Also, aberrations connected to marginal zone lymphoma were found, namely translocations t(14;18)(q32;q21) and t(3;14), trisomy 18, and deletion 7q. Age is a strong negative prognostic factor in all lymphoma subtypes [16]. Consistent with the research from 2017 [17], results of this study indicate that patients with NHL were predominantly males (56%). The lowest number of respondents were under the age of 30 years (6.25% for <20 and 6.25% for 21-30). The overall percentage of patients over 60 years was 31.25%.
In a 2012 study [18], isolated abnormalities that occurred in at least five MDS patients and were also observed during this research, included, among others, + mar, +1q, and +11. However, some of the most common cytogenetic aberrations, involving chromosomes 7, 8 and 17q, have not been reported in analysed patients. Since these aberrations are detected using classical cytogenetics in about 50% of MDS patients [19], it is possible that the use of complementary methods, such as fluorescent in situ hybridisation and comparative genomic hybridization, would enable more accurate results, because they allow the detection of chromosomal aberrations in an additional 20-40% of cases. MDS are generally diseases of the elderly, with a mean age at diagnosis of 65 to 70 years; less than 10% of patients are <50 years old. The disorder shows a slight predominance in men, except in the form of an isolated 5q deletion, in which it predominates in women [20]. The largest number of MDS patients recorded in this study (92.3%) was over 60 years of age. As observed in other investigations, men were slightly more often diagnosed with MDS (male: female ratio was 1.08: 0.92).
Aberrations consistent with previously published results of CLL cytogenetic studies, which were found in this examination, are translocations that include chromosomes 6, 3, and the presence of marker chromosomes. Other observed anomalies in assessed patients have not been found in the results of previous studies. However, CLL is a heterogeneous disease that does not have a characteristic mutation for the illness. The cytogenetic abnormalities most commonly present in CLL patients are deletions 13q, 11q22-23, and 17p13, trisomy 12, additional 2p and 3q; none of them were observed in this sample of subjects. Consistent with the literature, the mean age of analysed CLL subjects was 52.5 years, ranging from 16 to 81, with 38.5% of patients being over 60 years of age. The onset of CLL in the elderly can be attributed in part to the accumulation of toxic compounds in lymphoid or other interacting tissues as a result of chronic exposure to genotoxic agents, which can cause chromosomal aberrations and, in some cases, lead to carcinogenesis. In accordance with the 2019 study [21], male CLL patients predominated, with a male: female ratio of 1.24: 0.76.
Karyotype is necessary for diagnostic and prognostic purposes in patients with myeloproliferative neoplasms; cytogenetic abnormalities are detected in approximately 14-20% of polycythaemia vera, 30-50% of primary myelofibrosis, and 5%-10% of essential thrombocythaemia patients. There is no specific cytogenetic abnormality that determines MPN; however, there are several recurrent chromosomal aberrations that are associated with prognosis [22]. A normal karyogram was observed in 90.9% of MPN patients, which puts them in a favourable risk group, while 9.1% had structural and numerical chromosomal abnormalities, and a complex karyotype puts them in a high-risk group. Observed aberrations included deletions of 1q, 2p and 13, isochromosome 17, tumour marker type D, translocation t(3;21)(q26;q22), and a lack of Y chromosome.
Consistent with the available literature, the mean age of MPN subjects was 56.7 years, ranging from 28 to 70 years old (no age data was available for one patient). The majority of patients were male, with a male: female ratio of 1.82: 0.18.
Limitation(s)
Analysis of patient survival was not possible since many of them were not monitored during the course of their disease; even if they were scheduled to visit the centre again, they usually did not return for any further analysis.
Conclusion(s)
The study results show that the use of conventional cytogenetic analysis is a good diagnostic method for 50.68% of analysed subjects (74/146 patients) in whom chromosomal aberrations were observed. A number of novel (previously undescribed) chromosomal anomalies were discovered using conventional cytogenetic method, demonstrating its importance in the investigation of haematological neoplasms.