Introduction
Aluminium (AL) exposure leads to neurotoxicity and many problems in the body. AL role in Alzheimer’s Disease (AD) is unknown and controversial to the scientists. According to World Health Organisation (WHO) provisional tolerable weekly intake of AL is 2 mg/kg body weight. Moderate intake of alcohol may favour body in Coronary Heart Disease (CHD) and diabetes mellitus, etc. Aluminium being cheaper along with increased consumption of alcohol, mixed with each other may induce neurotoxicity.
Aim
To identify the effects of AL in cerebrum of rats in presence of ethanol co-exposure.
Materials and Methods
An experimental study was carried out at Dr. RP Government Medical College, Kangra and Government Medical College, Amritsar, India after due approval from the Institute Animal Ethics Committee. Thirty-two wistar rats were divided into one vehicle control and three experimental groups. Group I received the normal saline water as vehicle control group. Group II received AL chloride 4.2 mg/kg body weight as experimental group. Group III received ethanol 1 gm/kg body weight as experimental group. Group IV received both AL chloride 4.2 mg/kg body weight and ethanol 1 gm/kg body weight as experimental group. After treatment, brain cortex was processed for histopathological observation under microscope.
Results
Cerebral cortex showed normal architecture of the brain with Haematoxylin and Eosin (H&E) staining and cresyl violet staining and modified Bielschowsky’s silver staining in low and high magnification in vehicle control group. Experimental group treated with AL and ethanol separately showed reduction in the count of pyramidal cells with moderate neuronal degeneration with pyknotic nuclei. Vacuolar changes and pericellular spaces around the necrotic neurons were also seen. Combined AL and ethanol treated group showed acute neurodegeneration and necrosis of cortex indicating chromatolysis and loss of substances and Neurofibrillary Tangle (NFT) and plaque.
Conclusion
It has been concluded that the ethanol induced the effects of AL on the cerebrum and plays a significant role in AD pathogenesis.
Alcohol, Aluminium chloride, Alzheimer’s disease, Brain, Neurofibrillary tangle
Introduction
The AL is the 3rd most common element found in nature after silicon and oxygen, comprising approximately 8% of the earth crust [1]. Central Nervous System (CNS) is susceptible to AL toxicity and oxidative stress. It is well-documented that AL exposure causes neurotoxicity due to oxidative injury [2]. Many researches are conducted to find out the effects of AL toxicity on CNS. Due to lack of awareness of the toxic effects of AL and being cheap, AL utensils and containers are used more in India. AL is widely used and its consumption is noted through food, drinking water, AL containing drugs, containers, utensils and food wrappings [3]. AL intake varies upon place, country and diet. Approximately, 10 mg of AL is consumed daily by the human body. Out of 10 mg, 9.6 mg of AL is taken up from foods and 0.1-0.4 mg from utensils and 5 μg of AL taken from air [4]. AL is in very less amount and 95% of the total AL is excreted by feces [5]. Citrate may increase the AL absorption from the gastrointestinal tract. Malate, gluconate, lactate carbonate, ascorbate also facilitate the AL absorption [6]. It is also noted that silicone and iron decrease the absorption of AL and eliminate through urine and feces [7].
Alzheimer’s disease by a neurological progressive impairment affecting several cognitive domains behaviour, and personality [8]. AD is characterised by cerebral dysfunction along with biochemical alteration. As a result, leads to difficulties to understand previously known work and correlate new information, confusion, loss of memory and personality disorder [9]. NFTs due to hyperphosphorylation of tau protein and deposition of extracellular senile plaque due to hyper phosphorylation of amyloid beta protein are the most important pathological sign of AD. Loss of synapse and neurons, atrophy is also noted in AD [10,11]. Familial gene mutation may onset the AD in early life by altering the secretion of amyloid beta protein. Apolipoprotein E susceptibility gene is a risk factor in AD [12]. Many studies explained the role of AL in AD, Parkinsonism and Dementia. But the mechanism of AL neurotoxicity is not clear [13].
The cerebral cortex consists of six layers with pyramidal cells and neuroglial cells which functions in memory, awareness, attention, thought and language and consciousness. It also controls general movements, visceral function, behavioural function and mental function. Consumption of alcohol increased in the USA and other countries since last few years [14]. Alcohol alters the neurotransmitter and plasticity in prefrontal cortex, hippocampus and the limbic system structures [15]. Elizabeth R et al., explained that moderate alcohol consumption increase the HDL level and reduce the cholesterol level in the blood and reduce the risk of stroke and stress, anxiety, tension and AD also [16]. Ghosh B et al., demonstrated that early chronic ethanol exposure reduces the number of neuronal cells in the cerebellum [17]. Moderate intake of alcohol may control CHD in women with type 2 diabetes mellitus [18,19]. Excessive alcohol consumption causes impairment in cognitive and executive function and memory impairment and dementia. It also causes social problems including violence [20]. It is documented that AL toxicity may be influenced by ethanol co-exposure [21,22]. Moderate beer consumption may be a protecting factor for the toxic effects of AL [23,24]. It is noted that ethanol alters the pharmacokinetics of AL [7]. AL and alcohol exposure together enhanced the toxicity and neuropathological changes [25] and may induce the age-related deterioration in brain [26,27]. Effects of ethanol against the AL toxicity needs to be investigated. Present study was designed to find out the effects of AL on the microscopic structure of brain in presence of ethanol co-exposure.
Materials and Methods
An experimental study was carried out at Dr. RP Government Medical College, Kangra and Government Medical College, Amritsar, India from October 2014 to October 2018 after due approval from the Institute Animal Ethics Committee, Registration no 668/02/C/CPCSEA. Thirty-two wistar rats including equal number of male and female of an average age of 120 days and average weight of 200 gm were used in this study. After one week of acclimatisation rats were randomly divided into four groups containing eight animals (four male and four female) with the help of Random Allocation software version 1.0, May 2004. Animals were kept in the airconditioned animal house and fed with diet pellets and filtered tap water.
Group I received the normal saline water as vehicle control group.
Group II received AL chloride 4.2 mg/kg body weight as experimental group.
Group III received ethanol 1 gm/kg body weight as experimental group.
Group IV received both AL chloride 4.2 mg/kg body weight and also ethanol 1 gm/kg body weight as experimental group.
The treatments were carried out through oral feeding gavage once daily for period of 90 days. Their weights were recorded daily. After three months, the animals were anaesthetised with chloroform and an intracardiac perfusion of normal saline followed by 10% formaldehyde was performed. The brains of all groups of animals were extracted on an ice chilled tray. After sectioning, the cerebrum of all animals was processed for routine paraffin embedding.
Histological Study
The brains were removed and fixed in the 10% formalin solution for 24 hours. Following routine processing in paraffin, serial coronal sections of the brain were cut at 6 μm thickness in automated rotary microtome (Leica biosystem). The parts of each rat brain section were stained with H&E staining, cresyl violet staining and Bielschowsky’s silver staining according to Bancroft JD and Cook HC Theory and Practice of Histological Techniques [28].
For H&E staining, slide were deparaffinised through xylene (2-3 min) and absolute alcohol (1-2 min) then dipped in 95% alcohol followed by 70%, 50% and 30% alcohol. Then washed thoroughly with distilled water and placed in haematoxylin for 3-5 minutes and then examined the section after rinse with distilled water under low magnification of microscope to confirm its over stained. Then rinsed in distilled water and the slide was placed in another jar of 30% alcohol for three minutes. Then placed in 50% alcohol and followed by 70% alcohol and 95% alcohol. Then the slide was counter stained in 0.5-1% eosin in 90% alcohol for 30 seconds to one minute until the cytoplasm take deep pink stain. Then dipped in 95% alcohol for few seconds and placed in absolute alcohol for three minutes. To ensure full dehydration it was kept in next absolute alcohol for three minutes. Then the slide was transferred in xylene for 2 minutes and followed by next xylene for two minutes until the section appeared absolutely clear or transparent.
Cresyl violet staining slide were deparaffinised through xylene (2-3 min) and absolute alcohols (1-2 min) then dipped in 95% alcohol followed by 70%, 50%, 30% alcohol. Then placed in with 0.5% filtered Cresyl fast violet; stained for 10 minutes. And then rinsed with distilled water. Then the slide was differentiated in 0.25% acetic alcohol until most of the stain was removed (4-8 seconds). The slide was passed through the absolute alcohol into xylene and it was checked microscopically for appropriate staining. The slide was mounted in DPX after rinsing well in xylene.
Modified Bielschowsky’s silver staining demonstrates NFTs, nerve fibres and senile plaques in AD. For modified Bielschowsky’s silver staining the brain section of all the four groups were deparaffinised and hydrated with distilled water three minutes. Then, the slide was placed in prewarmed 20% silver nitrate at 40°C for 15 minutes. Section should turn light brown in colour. Then the section was placed in distilled water for three minutes. Ammonium silver solution was freshly prepared by adding concentrated ammonium hydroxide one drop at a time to the silver nitrate solution until the precipitate that forms clears. Excess ammonium hydroxide may cause a precipitate and result in a poor impregnation of the fibres. The solution was prepared just before use and discarded after use. The slide were incubated in the ammonium silver solution in a 40°C oven for 30 minutes until the section became dark brown. A developer stock solution was prepared by adding formaldehyde 20 mL, citric acid 0.5 gm, nitric acid two drops, distilled water 100 mL. Eight drops of the above developer stock solution were added to the developer working solution (distilled water 50 mL and ammonium hydroxide eight drops). The slide was then placed to developer working solution for two to five minutes. The staining reaction occurred very quickly and and it was confirmed by viewing the slide under microscope. The slides were dipped in the 1% ammonium hydroxide solution (0.5 mL concentrated ammonium hydroxide in 50 mL distilled water) to stop the silver reaction if needful. Slide were washed in distilled water three minutes. And then slide were placed in 5% sodium thiosulfate solution for five minutes. Again, washed in distilled water three minutes and dehydrated with absolute alcohol and cleared with xylene and mounted with DPX. The slide was viewed under microscope. The histopathological observation were analysed by seeing the morphological changes in neuronal cells and parenchyma in low and high magnification.
Statistical Analysis
Descriptive statistics were used to analyse the data.
Results
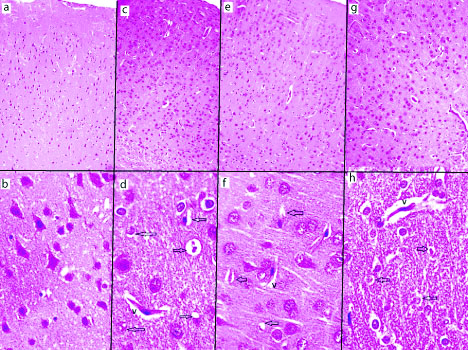
Cerebrum with H&E staining showed the normal architecture in vehicle control (group I) in [Table/Fig-1a,b] in low and high magnification. The six layers of cerebrum are seen as molecular layer, external granular layer, external pyramidal layer, internal granular layer, internal pyramidal layer and pleiomorphic layer in low magnification. Large pyramidal cells were seen in internal pyramidal layer in high magnification. In all these layers, the neurons appeared with large nuclei and basophilic cytoplasm while smaller glia cells were scattered in between neurons. The AL treated group (group II) showed moderate neurodegeneration of pyramidal cells and vacuolar changes with few empty spaces and pericellular spaces around the necrotic neurons [Table/Fig-1c,d]. The ethanol treated (group III) showed various histopathological changes and moderate neuronal degeneration with pyknotic nuclei [Table/Fig-1e,f]. The AL and ethanol combined treated (group IV) showed acute neurodegeneration and necrosis of cerebral cortex, indicating vacuoles, pericellular spaces around the necrotic neurons [Table/Fig-1g,h].
H&E staining. Low magnification (10x) and high magnification (40x). Vehicle control group cerebral cortex (a and b), aluminium treated group cerebral cortex (c & d), ethanol treated group cerebral cortex (e and f), combined aluminium ethanol treated group cerebral cortex (g and h). a) The six layer of cerebrum seen as molecular layer, external granular layer, external pyramidal layer, internal granular layer, internal pyramidal layer and pleiomorphic layer (10x). b) Pyramidal cell seen in high magnification (40x). c) diffuse neuronal cell loss (10x). d) vacuolisation (v) and astrocytosis and pericellular spaces around the necrotic neurons (black arrow) (40x). e) diffuse neuronal loss (10x). f) pericellular spaces around the necrotic neurons (black arrow) (40x). g) diffuse neuronal loss (10x). h) pyknotic neurons and pericellular spaces around the necrotic neurons (black arrow) (40x).
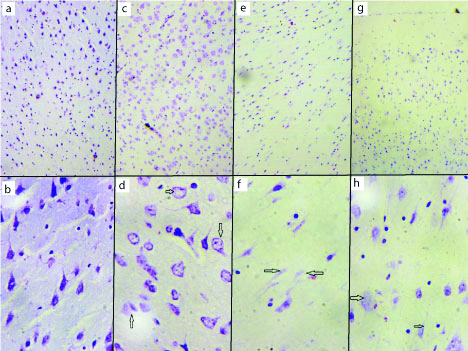
Cerebrum with cresyl violet staining showed the normal architecture of the brain in vehicle control (group I) as shown in [Table/Fig-2a,b]. The AL treated group (group II) showed moderate neurodegeneration and loss of nissl substances [Table/Fig-2c,d]. The ethanol treated (group III) showed various histopathological changes in the pyramidal cells with necrosis of the pyramidal cells [Table/Fig-2e,f]. The AL and ethanol combined treated (group IV) showed acute neurodegeneration and necrosis of cerebral cortex, indicating vacuoles, pericellular spaces around the necrotic neurons and chromatolysis [Table/Fig-2g,h].
Cresyl violet staining. Low magnification (10x) and high magnification (40x). Vehicle control group cerebral cortex (a and b), aluminium treated group cerebral cortex (c and d), ethanol treated group cerebral cortex (e and f), combined aluminium ethanol treated group cerebral cortex (g and h). a) The six layers of cerebrum seen (10x). b) Pyramidal cell seen in high magnification (40x). c) diffuse neuronal cell loss (10x). d) chromatolysis and loss of nissl substance in the pyramidal cell (arrow) (40x). e) diffuse neuronal loss (10x). f) necrotic neurons (arrow) (40x). g) diffuse neuronal loss (10x). h) chromatolysis and loss of nissl substance in the pyramidal cell (40x).
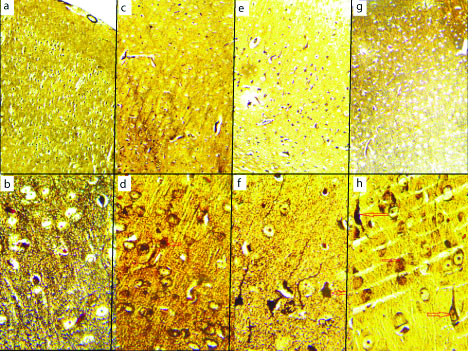
Cerebrum with modified Bielschowsky’s Silver staining showed the normal architecture of the brain in vehicle control (group I) [Table/Fig-3a,b]. The AL treated group (group II) showed moderate neurodegeneration of pyramidal cells [Table/Fig-3c,d]. The ethanol treated (group III) showed various histopathological changes and moderate neuronal degeneration with pyknotic nuclei [Table/Fig-3e,f]. The AL and ethanol combined treated (group IV) showed acute neurodegeneration of cerebral cortex, several neuronal loss with NFT and plaque seen in [Table/Fig-3g,h].
Modified Bielchowsky silver staining. Low magnification (10x) and high magnification (40x). Vehicle control group cerebral cortex (a and b), aluminium treated group cerebral cortex (c and d), ethanol treated group cerebral cortex (e and f), combined aluminium ethanol treated group cerebral cortex (g and h). a) The six layers of cerebrum seen (10x). b) Pyramidal cell and axon seen in high magnification (40x). c) diffuse neuronal cell loss (10x). d) necrotic pyramidal cells (40x). e) diffuse neuronal loss (10x). f) loss of pyramidal cells (40x). g) Severe neuronal loss (10x). h) Neurofibrillary Tangle and plaque (arrow) (40x).
Discussion
The AL crosses the blood brain barrier and effects the brain [29]. High dose of AL leads to neurodegeneration, NFT and senile plaque formation in the brain. AL accumulate in the large pyramidal neurons of the cortex, hippocampus and other region of the brain and leads to AD [30]. Klotz K et al., explained provisional tolerable weekly intake is 2 mg AL/kg body weight. Biological tolerance value at the workplace derived on the basis of neurotoxicity as the critical endpoint 50 μg/g creatinine (BAT value); not classified into a carcinogenicity category according to German Research Foundation. The tolerable weekly intake set by the European Food Safety Authority (EFSA) is 1 mg AL/kg body weight which can be reached through dietary exposure alone [31]. In the present study, neurodegeneration of pyramidal cells in cortex with moderate vacuolar changes with few empty spaces were seen. Pericellular spaces around the necrotic neurons were also seen due to moderate AL toxicity. Previous study explained AD brains contain higher levels of AL. Spectrograph study explained that the AL content is more in AD brain than normal brain [32]. AD is a neurological disorder due to formation of senile plaques, NFT and loss of cholinergic neurons in the brain [33]. Accumulation of AL in brain and senile plaque and NFT is also observed in AD [34].
Researchers in 1965 found tau tangles and amyloid levels in the brains of rabbits after AL exposure. Afterwards researchers focused on the relationship with AL and AD [35]. Few researches explained that oxidative stress also induces AD [36]. But direct role of AL in AD has not been established. Epidemiological studies suggest that AL level may link with AD. The main pathological features of the AD are the deposition of amyloid beta and senile plaque and NFT [37]. AL induce gliosis and such neurodegenerative condition which can also be seen in AD and Parkinson’s disease [38]. This neuronal loss in AD is due to gliosis and oxidative stress [39]. Neurodegeneration and decrease in brain volume are due to the loss of axon and synapse and neurons [40].
Binge alcohol consumption may induce AD [41]. The amount or dose of alcohol developing AD is not clear till date. In this study, it was found that found ethanol induced various histopathological changes like neuronal degeneration with pyknotic nuclei compared with control group. Cholinergic system is affected by both alcohol and AL. Therefore, alcohol may induce the AD. Early study explained cholinergic system and its role in memory and also in AD. Lower levels of acetylcholine are found in AD [42]. Alcohol also effects on many neurotransmitter and induced AD [43]. One study explained the protective effects of alcohol in AD [44]. Epidemiological studies didn’t find any correlation between alcohol use and AD. Loss of neurons connection with cholinergic neurons is documented in AD [45]. Humans and animals are affected more in exposure to mixture of toxic agents [46]. Brain damage in rats were significantly noted with AL and acrylamide exposure. However, when the animals were exposed to ethanol and AL the brain tissue became more affected, especially with the higher doses of ethanol. Ethanol induces the oxidative stress alone in the brain but AL with pro-oxidant ethanol favour the AL induced oxidative stress in cerebrum [47]. In the present study, AL and ethanol combined treated brain showed chronic neurodegeneration and necrosis of cerebral cortex, indicating vacuoles, pericellular spaces around the necrotic neurons, chromatolysis and loss of nissl substances. Neuritic plaque and tangle were also seen in the cerebrum. Moreover, the present data demonstrated that chronic ethanol exposure induces the AL toxicity in cerebral cortex and plays a significant role in AD pathogenesis.
Limitation(s)
In addition to gender, different age group of rats may be included in the study. This study explained only histopathological changes of the brain structure under microscope with special staining. More extensive work in the molecular level and statistical explanation is required.
Conclusion(s)
Present study showed that AL and ethanol both are neurotoxicant. Most importantly NFT and plaque were seen in combined exposure of AL and ethanol treatment. It has been suggested that the ethanol induced the effects of AL on the cerebrum and plays a significant role in AD pathogenesis.
[1]. Elif E, Aylin A, Is aluminium exposure a risk factor for neurological disorders?J Res Med Sci 2018 23:5110.4103/jrms.JRMS_921_1730057635 [Google Scholar] [CrossRef] [PubMed]
[2]. Nayak P, Aluminium induced intensification of oxidative stress in ethanol exposed brain: A dose-dependent study on rat brainJ Environ Physiol 2009 2(1.2):61-72. [Google Scholar]
[3]. Karbouj R, Aluminium leaching using chelating agents as compositions of foodFood Chem Toxicol 2007 45:1688-93.10.1016/j.fct.2007.03.00117434655 [Google Scholar] [CrossRef] [PubMed]
[4]. Vargel C, Food Industry 2004 st edOxfordElseiver10.1016/B978-008044495-6/50043-4 [Google Scholar] [CrossRef]
[5]. Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brainHistol Histopathol 2008 23:433-39. [Google Scholar]
[6]. Glynn AW, Sparen A, Danielsson LG, Sundström B, Jorhem L, The influence of complexing agents on the solubility and absorption of aluminium in rats exposed to aluminium in waterFood Addit Contam 2001 18:515-23./10.1080/0265203011863911407750 [Google Scholar] [CrossRef] [PubMed]
[7]. Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxideJ Toxicol Environ Health B Crit Rev 2007 10(1):01-269.10.1080/1093740070159776618085482 [Google Scholar] [CrossRef] [PubMed]
[8]. Wang Z, Wei X, Yang J, Suo J, Chen J, Liu X, Chronic exposure to aluminium and risk of Alzheimer’s disease: A meta-analysisNeurosci Lett 2016 610:200-06.10.1016/j.neulet.2015.11.01426592479 [Google Scholar] [CrossRef] [PubMed]
[9]. Pedersen NL, Reaching the limits of genome-wide significance in Alzheimer disease: Back to the environmentJAMA 2010 303:1864-65.10.1001/jama.2010.60920460629 [Google Scholar] [CrossRef] [PubMed]
[10]. Kawahara M, Effects of aluminium on the nervous system and its possible link with neurodegenerative diseasesJ Alzheimers Dis 2005 8:171-82.10.3233/JAD-2005-821016308486 [Google Scholar] [CrossRef] [PubMed]
[11]. Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R, Aluminium in Alzheimer’s disease: Are we still at a crossroad?Cell Mol Life Sci 2005 62:143-58.10.1007/s00018-004-4317-315666086 [Google Scholar] [CrossRef] [PubMed]
[12]. Ferreira PC, PiaiKde A, Takayanagui AM, Segura-Munoz SI, Aluminium as a risk factor for Alzheimer’s diseaseRev Lat Am Enfermagem 2008 16:151-57.10.1590/S0104-1169200800010002318392545 [Google Scholar] [CrossRef] [PubMed]
[13]. Niu Q, Yang Y, Zhang Q, Niu P, He S, Di Gioacchino M, The relationship between Bcl-2 gene expression and learning & memory impairment in chronic aluminium exposed ratsNeurotox Res 2007 12(3):163-69.10.1007/BF0303391317967740 [Google Scholar] [CrossRef] [PubMed]
[14]. Kim JH, Prevalence and the factors associated with binge drinking, alcohol abuse, and alcohol dependence: A population-based study of Chinese adults in Hong KongAlcohol 2008 43:360-70.10.1093/alcalc/agm18118230698 [Google Scholar] [CrossRef] [PubMed]
[15]. Pagey B, Deering D, Sellman D, Retention of adolescents with substance dependence and coexisting mental health disorders in outpatient alcohol and drug group therapyInt J Ment Health Nurs 2010 19:437-44.10.1111/j.1447-0349.2010.00693.x21054730 [Google Scholar] [CrossRef] [PubMed]
[16]. Elizabeth R, Silva DO, Foster D, Harper MM, Seidman CE, Alcohol consumption raises HDL Cholesterol Levels by Increasing the Transport Rate of Apolipoproteins A-I and A-IICirculation 2000 102:2347-52.10.1161/01.CIR.102.19.234711067787 [Google Scholar] [CrossRef] [PubMed]
[17]. Ghosh B, Sharma R, Yadav S, Parashar V, Jagdish P, Ethanol exposure induces cerebellar neuronal loss in ratsEur J Anat 2020 24(5):407-13. [Google Scholar]
[18]. Regan TJ, Moderate alcohol consumption and risk of coronary heart disease among women with type 2 diabetes mellitusCirculation 2000 102:487-88.10.1161/01.CIR.102.5.48710920056 [Google Scholar] [CrossRef] [PubMed]
[19]. Koppesl J, Moderate alcohol consumption lowers the risk of type 2 diabetesDiabetes Care 2005 28:719-25.10.2337/diacare.28.3.71915735217 [Google Scholar] [CrossRef] [PubMed]
[20]. Kuzmin A, Chefer V, Upregulated dynorphin opioid peptides mediate alcohol-induced learning and memory impairmentTransl Psychiatry 2013 3:7210.1038/tp.2013.7224105441 [Google Scholar] [CrossRef] [PubMed]
[21]. Nayak P, Das SK, Vasudevan DM, Role of ethanol on aluminium induced biochemical changes on rat brainIndian Journal of Clinical Biochemistry 2006 21(2):53-57.10.1007/BF0291291223105614 [Google Scholar] [CrossRef] [PubMed]
[22]. Nayak P, Sharma SB, Chowdary NV, Aluminium and ethanol induce Alterations in superoxide and peroxide handling capacity (SPHC) in frontal and temporal cortexIndian J Biochem Biophys 2013 50(5):402-10. [Google Scholar]
[23]. Pena A, Meseguer I, Influence of moderate beer consumption on aluminiumtoxico-kynetics: Acute studyNutr Hosp 2007 22(3):371-76. [Google Scholar]
[24]. Gonzalez Munoz MJ, Role of beer as a possible protective factor in preventing Alzheimer’s diseaseFood Chem Toxicol 2008 46(1):49-56.10.1016/j.fct.2007.06.03617697731 [Google Scholar] [CrossRef] [PubMed]
[25]. Castellani RJ, Zhu X, Lee HG, Smith MA, Perry G, Molecular pathogenesis of Alzheimer’s disease: Reductionist versus expansionist approachesInt J Mol Sci 2009 10:1386-406.10.3390/ijms1003138619399255 [Google Scholar] [CrossRef] [PubMed]
[26]. Dlugos CA, Ethanol-related increases in degenerating bodies in the purkinje neuron dendrites of aging ratsBrain Res 2008 1221:98-107.10.1016/j.brainres.2008.05.01518559274 [Google Scholar] [CrossRef] [PubMed]
[27]. Tripathi S, Mahdi AA, Nawab A, Influence of age on aluminium induced lipid peroxidation and neurolipofusci in frontal cortex of rat brain: A behavioral, biochemical and ultrastructural studyBrain Res 2009 1253:107-16.10.1016/j.brainres.2008.11.06019073157 [Google Scholar] [CrossRef] [PubMed]
[28]. Bancroft JD, Cook HC, Manual of Histological Techniques 1984 EdinburghChurchill Livingstone:201-202. [Google Scholar]
[29]. Mooradian AD, Effect of aging on the blood-brain barrierNeurobiol Aging 1988 9:31-39.10.1016/S0197-4580(88)80013-7 [Google Scholar] [CrossRef]
[30]. Walton JR, Aluminum disruption of calcium homeostasis and signal transduction resembles change that occurs in aging and Alzheimer’s diseaseJ Alzehimer Disease 2012 29(2):255-73.10.3233/JAD-2011-11171222330830 [Google Scholar] [CrossRef] [PubMed]
[31]. Klotz K, Weistenhofer W, Neff F, Hartwig A, van Thriel C, Drexler H, The health effects of aluminum exposureDtsch Arztebl Int 2017 114:653-59.10.3238/arztebl.2017.065329034866 [Google Scholar] [CrossRef] [PubMed]
[32]. Fattoretti P, Bertoni-Freddari C, Balietti M, Giorgetti B, Solazzi M, Zatta P, Chronic aluminum administration to old rats results in increased levels of brain metalions and enlarged hippocampal mossy fibersAnn N Y Acad Sci 2004 1019(8):44-47.10.1196/annals.1297.01015246992 [Google Scholar] [CrossRef] [PubMed]
[33]. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR, Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrainScience 1982 215(4537):1237-39.10.1126/science.70583417058341 [Google Scholar] [CrossRef] [PubMed]
[34]. Rusina R, Matej R, Kasparov A, Kukal J, Urban P, Higher aluminum concentration in Alzheimer’s disease after Box- Cox data transformationNeurotox Res 2011 20:329-33.10.1007/s12640-011-9246-y21567285 [Google Scholar] [CrossRef] [PubMed]
[35]. Miu AC, Benga O, Aluminum and Alzheimer’s disease: A new lookJ Alzheimers Dis 2006 10:179-201.10.3233/JAD-2006-102-30617119287 [Google Scholar] [CrossRef] [PubMed]
[36]. Bondy SC, Campbell A, Initiation of futile pathways common to many neurological diseases. In: Marwah J, Lo E, edsNeuroprotection In press, 2001 Prominent Press [Google Scholar]
[37]. Medeiros R, LaFerla FM, Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphonyExp Neurol 2013 239:133-38.10.1016/j.expneurol.2012.10.00723063604 [Google Scholar] [CrossRef] [PubMed]
[38]. Shahripour RB, Harrigan MR, Alexandrov AV, N-acetyl cysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunitiesBrain and Behavior 2014 4(2):108-22.10.1002/brb3.20824683506 [Google Scholar] [CrossRef] [PubMed]
[39]. Wang Q, Liu Y, Zhou J, Neuroinflammation in Parkinson’s disease and its potential as therapeutic targetTransl Neurodegener 2015 4(1):1910.1186/s40035-015-0042-026464797 [Google Scholar] [CrossRef] [PubMed]
[40]. Miller RL, James-Kracke M, Sun GY, Sun AY, Oxidative andinflammatory pathways in Parkinson’s diseaseNeurochem Res 2009 34(1):55-65.10.1007/s11064-008-9656-218363100 [Google Scholar] [CrossRef] [PubMed]
[41]. KaddourTair OK, Oussama A, Nouria H, Imene B, Abdelkader A, Aluminum-induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammadascoparia)Journal of Acute Disease 2016 5(6):470-82.10.1016/j.joad.2016.08.028 [Google Scholar] [CrossRef]
[42]. Tyas SL, Are tobacco and alcohol use related to Alzheimer’s disease? A critical assessment of the evidence and its implicationsAddiction Biology 1996 1:237-54.10.1080/135562196100012485612893463 [Google Scholar] [CrossRef] [PubMed]
[43]. Forette F, Boller F, Trends in Alzheimer’s disease treatment and prevention. In: Iqbal K, Swaab DF, Winblad B, Wisniewski HMAlzheimer’s Disease and Related Disorders: Etiology, Pathogenesis, and Therapeutics 1999 ChichesterJohn Wiley & Sons:623-31. [Google Scholar]
[44]. Callahan CM, Hall KS, Hui SL, Musick BS, Unverzagt FW, Hendrie HC, Relationship of age, education, and occupation with dementia among a community-based sample of African AmericansArchives of Neurology 1996 53:134-40.10.1001/archneur.1996.005500200380138639062 [Google Scholar] [CrossRef] [PubMed]
[45]. Cupples LA, Weinberg J, Beiser A, Auerbach SH, Volicer L, Cipolloni PB, Effects of smoking, alcohol and APOE genotype on Alzheimer disease: The MIRAGE studyAlzheimer’s Reports 2000 3(2):105-14.10.1016/S0197-4580(00)82828-6 [Google Scholar] [CrossRef]
[46]. Jensen GB, Pakkenberg B, Do alcoholics drink their neurons away?Lancet 1993 342:1201-04.10.1016/0140-6736(93)92185-V [Google Scholar] [CrossRef]
[47]. Ghorbel I, Amara IB, Ktari N, Elwej A, Boudawara O, Boudawara T, Zeghal N, Aluminium and Acrylamide disrupt cerebellum redox states, cholinergic function and membrane bound ATPase in adult rats and their offspringBiol Trace Elem Res 2016 174:335-46.10.1007/s12011-016-0716-127116954 [Google Scholar] [CrossRef] [PubMed]