Tzanck smear is a reliable, simple and cost-effective cytological technique. It has useful application in the diagnosis of vesiculobullous lesions, infective lesions, genodermatosis and cutaneous tumours [1]. The Tzanck smear uses the principle of exfoliative cytology to make the diagnosis. The procedure serves to take multiple samples from different lesions and areas where biopsy may be difficult to perform [2]. It has been regarded as an effective and fairly accurate cytodiagnostic tool [3].
There is paucity of studies on the utility of Tzanck smears from India. Being a rapid and simple diagnostic tool, further studies may help in validating the Tzanck smear as an investigative procedure in routine practice [7]. Most of the studies have focused only on describing the cytomorphological features and diagnosis of various dermatological lesions [8-11]. These studies have not focused on evaluating the staining characteristics of the stain used to study the Tzanck smear cytology.
The present study is a novel one in that it evaluates and highlights the staining characteristics of PAP and MCG stain in Tzanck smear cytology. The objectives of the present study were to study the cytomorphological features of various dermatological lesions and to compare the staining characteristics of PAP stained and MGG stained smears by using a scoring system.
Materials and Methods
The study was conducted at Cytopathology section in the Department of Pathology in coordination with the Department of Dermatology. It was cross-sectional study of staining characteristics of different staining methods on Tzanck smears, conducted from March 2016 to April 2017 at a rural tertiary care referral Institute, PES Institute of Medical Sciences and Research (PESIMSR), Kuppam, Andhra Pradesh, India. The study was approved by Institutional Ethics Committee bearing number PESIMSR/IHEC/42.
Sample size calculation: The sample size was estimated to be 37 cases. The sample size was calculated using following formula:
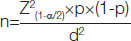
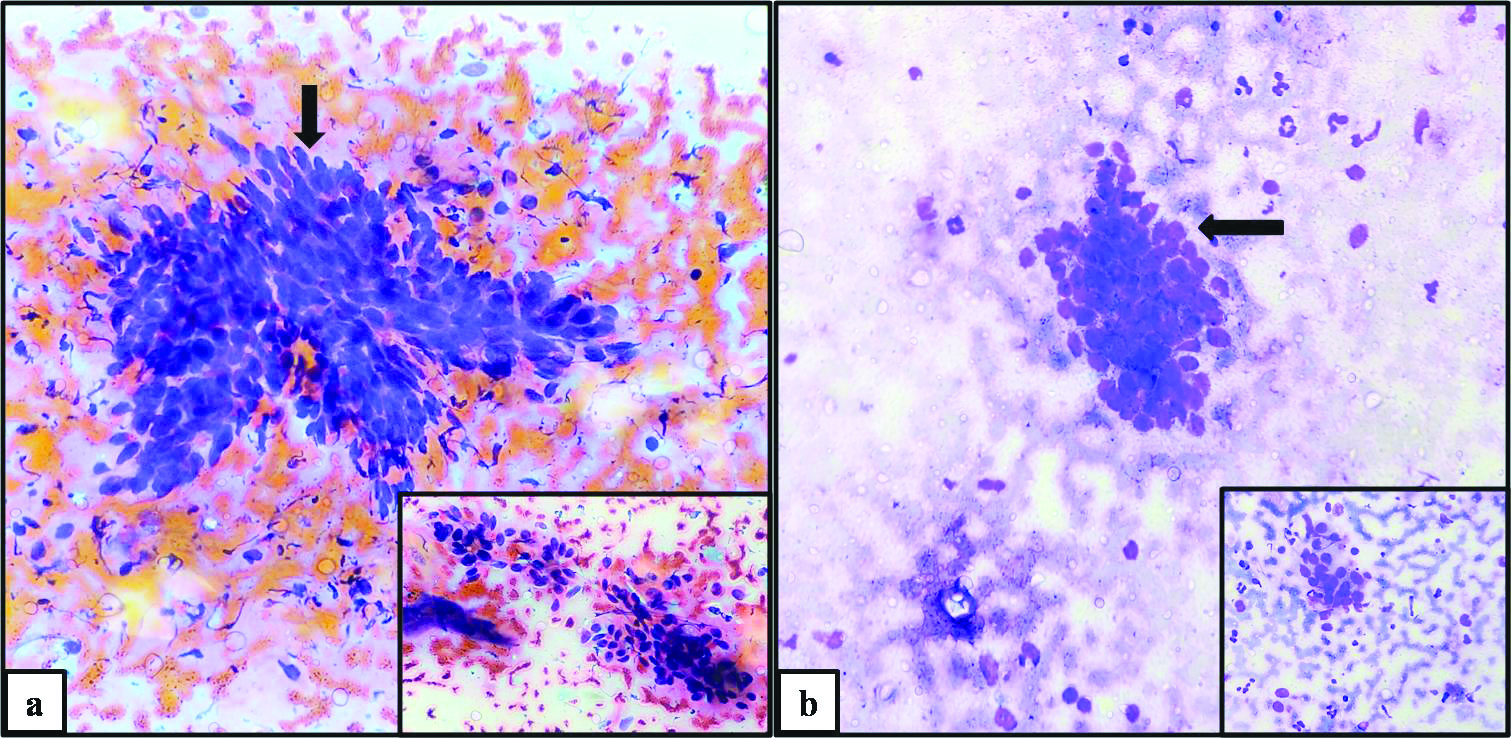
“n” is the sample size
“Z2(1-α/2)” is the level of significance at 5% that is 95% confidence interval
“p” is the expected proportion of cytology samples
“d” is the desired error of margin.
Inclusion criteria: Forty cases were analysed. All cases presenting with dermatological lesions requiring cytopathological evaluation were included in the study.
Exclusion criteria: Those cases in which the material obtained was inadequate to employ both the staining techniques were excluded from the study.
Study Procedure
In each case, two Tzanck smears were prepared from the skin lesions according to the standard operating procedure. One smear was fixed in isoproplyl alcohol and stained by PAP method (using staining kit from a standard company). Other smear was air dried and subsequently stained by MGG stain (using staining kit from a standard company). The staining procedure was done according to the standard operating procedure. Both the smears were evaluated for staining characteristics by using a scoring system [Table/Fig-1]. The scoring system was indigenously designed for evaluating the stained smears based on the parameters documented by Moosa NY et al., [12]. All the staining parameters such as contrast at low power (100X), cytoplasmic features, nuclear features and background were evaluated in the scoring system employed in the present study. Hence the present scoring system was considered reliable and suitable.
Scoring system employed for different stains on Tzanck smear.
| Effect of staining method on contrast at low power |
|---|
| Score 1: Very poor- unsuitable for assessment | Score 3: Optimal contrast features |
| Score 2: Sub-optimal- just acceptable for assessment | Score 4: Excellent contrast |
| Effect of staining method on cytoplasmic features |
| Score 1: Very poor- unsuitable for assessment | Score 3: Optimal cytoplasmic features |
| Score 2: Sub-optimal- just acceptable for assessment | Score 4: Excellent and sharp cytoplasmic features |
| Effect of staining method on nuclear features |
| Score 1: Very poor- unsuitable for assessment | Score 3: Optimal nuclear features |
| Score 2: Sub-optimal- just acceptable for assessment | Score 4: Excellent and sharp nuclear features |
| Effect of staining method on background features |
| Score 1: Very poor- unsuitable for assessment | Score 3: Optimal background features |
| Score 2: Sub-optimal- just acceptable for assessment | Score 4: Excellent background features |
Statistical Analysis
Chi-square test or Fischer exact test was applied for categorical variables and two sample t-test was applied for continuous variables to calculate the statistical difference in values. All results were analysed by considering the statistical significance at a level of p-value <0.05. All statistical calculations were done through statistical software STATA version 14.1.
Results
In the present study, 40 cases of Tzanck smears were analysed. Clustering of cases was seen in third decade (mean=31.07 years). The lesions were seen predominantly in females 21 cases (52.5%) with a M:F ratio of 0.9:1. Face was the most common site of involvement constituting 14 cases (35%), followed by upper extremity 13 cases (32.5%), lower extremity 4 cases (10%), trunk 3 cases (7.5%), external genitals 3 cases (7.5%) and chest 3 cases (7.5%).
Cytological Diagnosis
Most common diagnostic entity was cutaneous infections 18 cases (45%), followed by non specific inflammatory lesions 16 cases (40%), immuno-bullous disorders 3 cases (7.5%), non-diagnostic cases 2 cases (5%) and neoplastic lesion 1 case (2.5%) [Table/Fig-2].
Distribution of spectrum of cytopathology diagnoses of skin lesions on Tzanck smear.
| Cytopathology diagnoses | Cases (n=40) |
|---|
| Cutaneous infections | 18 (45%) |
| Herpes viral infections | 10 (25%) |
| Herpes viral infection with fungal infection | 2 (5%) |
| Fungal infection | 2 (5%) |
| Molluscum contagiosum | 2 (5%) |
| Bullous impetigo | 2 (5%) |
| Non-specific inflammatory lesions | 16 (40%) |
| Acute on chronic inflammatory lesions | 8 (20%) |
| Acute inflammatory lesions | 7 (17.5%) |
| Chronic inflammatory lesion | 1 (2.5%) |
| Immunobullous lesions | 3 (7.5%) |
| Pemphigus group (Pemphigus foliaceous) | 1 (2.5%) |
| Staphylococcal scalded skin syndrome | 1 (2.5%) |
| Bullous pemphigoid | 1 (2.5%) |
| Neoplastic lesion | 1 (2.5%) |
| Basal cell carcinoma | 1 (2.5%) |
| Non-diagnostic | 2 (5%) |
| Haemorrhagic material | 2 (5%) |
Cutaneous Infections
Herpes viral infections: Smears studied showed numerous acantholytic cells. The acantholytic cells were large to medium sized, rounded up cells having scant to moderate amount of cytoplasm, perinuclear halo peripheral cytoplasmic condensation and enlarged vesicular nuclei. Amidst the acantholytic cells, good number of multinucleated giant cells was seen. The giant cells were large, oval to tadpole shaped cells having moderate amount of cytoplasm, variable number of nuclei and perinuclear halo. The nuclei showed nuclear enlargement, nuclear molding, intra-nuclear inclusions and ground glass appearance. Also seen were few acute inflammatory cells. Background showed proteinaceous material [Table/Fig-3a,b].
Cutaneous infections: Herpes viral infection: Acantholytic cells (arrow) and Multinucleated giant cells (Inset) in PAP (a) and MGG (b) (400X). Fungal infection: Multinucleated giant cells (arrow) and fungi (arrowhead) [Inset] in PAP (c) and MGG (d) (400X). Molluscum Contagiosum: Molluscum bodies (arrow). Inset: Individual molluscum body in PAP(e) and MGG (f) (400X).
Fungal infections: Smears studied showed abundant inflammatory cells composed of predominantly neutrophils, few lymphocytes. Also seen were a few number of multinucleated giant cells (Langhans type and foreign body type) were seen. The giant cells were large, round to oval shaped cells having moderate amount of cytoplasm, variable number of nuclei. The nuclei showed nuclear enlargement, nuclear overlapping. Amidst the inflammatory cells, yeast forms of fungal elements (resembling Candida species) were seen. Background showed proteinaceous material [Table/Fig-3c,d].
Molluscum contagiosum: Smears studied showed few mature squamous epithelial cells. Amidst the epithelial cells, few large, ovoid, hyaline, vitreous and homogeneous inclusion bodies (Henderson-Patterson bodies) with eccentrically placed nucleus were seen. Background showed proteinaceous material [Table/Fig-3e,f].
Bullous impetigo: Smears studied showed abundant neutrophils and few lymphocytes. Amidst the inflammatory cells, dyskeratotic acantholytic cells were seen. Background showed coccoid bacteria and proteinaceous material.
Non-specific Inflammatory Lesions
Acute on chronic inflammatory lesion: Smears studied showed abundant neutrophils and few lymphocytes. Amidst the inflammatory cells, nucleated mature squamous epithelial cells and few anucleated squames were seen. No acantholytic cells were seen in the smears studied. Background showed proteinaceous material.
Acute inflammatory lesion: Smears studied showed abundant neutrophils. Amidst the inflammatory cells, nucleated mature squamous epithelial cells and few anucleated squames were seen. No acantholytic cells were seen in the smears studied. Background showed proteinaceous material.
Chronic inflammatory lesion: Smears studied showed numerous lymphocytes. Amidst the inflammatory cells, nucleated mature squamous epithelial cells and few anucleated squames were seen. No acantholytic cells were seen in the smears studied. Background showed proteinaceous material.
Immunobullous Lesions
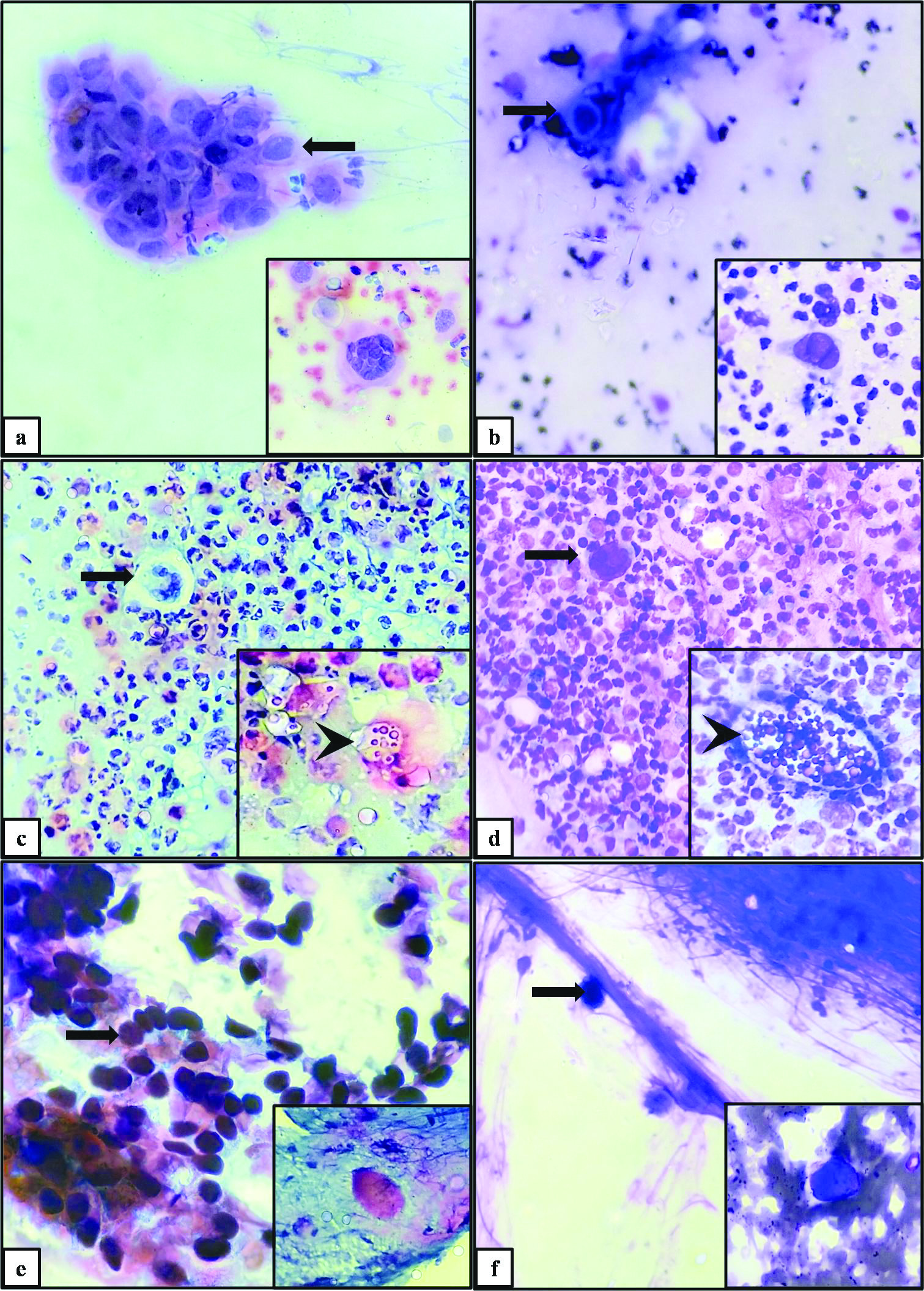
Pemphigus group (Pemphigus foliaceous): Smears studied showed numerous acantholytic cells. The acantholytic cells were large to medium sized, rounded up cells having scant to moderate amount of hyalinised cytoplasm, scant peinuclear halo, peripheral cytoplasmic condensation, enlarged vesicular nuclei. Amidst the acantholytic cells, occassional multinucleated giant cells were seen. Also seen were few acute inflammatory cells. Background showed proteinaceous material [Table/Fig-4].
Immunobullous lesions: Pemphigus foliaceous: Acantholytic cells (arrow) displaying hyalinised cytoplasm amidst the polymorphs in PAP (a) and MGG (b) (400X). Staphylococcal scalded skin syndrome: Dyskeratotic acantholytic cells (arrow) in a background of very scant inflammation in PAP (c) and MGG (d) (400X).
Staphylococcal scalded skin syndrome: Smears studied showed abundant dyskeratotic acantholytic cells. Amidst the acantholytic cells, few inflammatory cells were seen. Background showed proteinaceous material [Table/Fig-4].
Bullous pemphigoid: Smears studied showed abundant neutrophils and few lymphocytes. At places, acute inflammatory cells were arranged in chains (streptocytes). But, only occasional eosinophils were seen. Amidst the inflammatory cells, occasional squamous epithelial cells were seen. No acantholytic cells were seen in the smears studied. Background showed proteinaceous material. Possibility of vesicular variant were considered.
Neoplastic Lesion
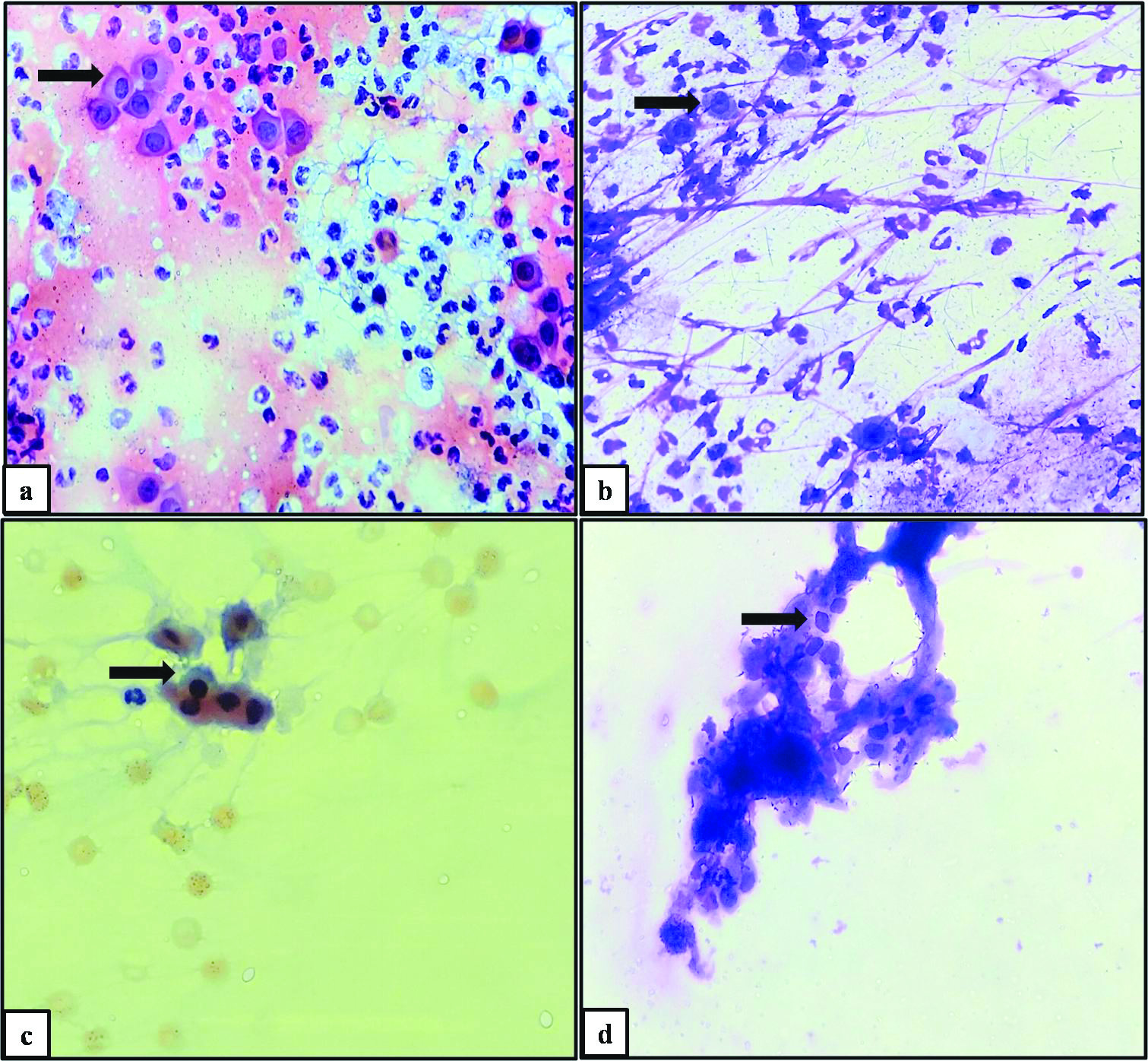
Basal cell carcinoma: Smears studied show tumour cells arranged in clusters. Individual clusters showed peripheral palisading of tumour cells. Individual tumour cells were medium sized basaloid cells having scant to moderate amount of deeply basophilic cytoplasm and elongated to oval, centrally placed intensely basophilic nucleus. Background showed proteinaceous material [Table/Fig-5].
Neoplastic lesion: Basal cell carcinoma: Basaloid tumour cells arranged in tight clusters displaying peripheral palisading (arrow) and few loosely cohesive cluster (Inset) in PAP (a) and MGG (b) (400X).
Non-diagnostic
Smears studied show only haemorrhagic material. Amidst the elements of blood, only occasional squamous epithelial cells were seen.
Histopathology
Histopathology could be performed in only three cases. In all the three cases, cytological diagnosis [pemphigus group (pemphigus folicaceous), Acute inflammatory lesion and chronic inflammatory lesion] was concordant with the final histopathlogical diagnosis (pemphigus folicaeous, acute on chronic inflammatory lesion and borderline tuberculoid leprosy).
Comparison of Effect of Staining on Tzanck Smears
Isopropyl alcohol fixed Tzanck smears stained by PAP and air dried Tzanck smears stained by MGG stain were examined, evaluated and compared for the quality of staining.
Contrast for differentiating between different cells or structures (inclusion bodies/fungi) was better appreciated in PAP stained smears [37 cases (92.5%) with score 3] than MGG stained smears [3 cases (7.5%) with score 3]. The p-value was highly significant (p<0.001). Average score of PAP stained smears (3.03) was better than MGG stained smears (2.08). The p-value was highly significant (p<0.001).
Cytoplasmic staining was better in PAP stained smears [39 cases (97.5%) with score 3] than MGG stained smears [33 cases (82.5%) with score 3]. The p-value was statistically significant (p=0.014). Average score of PAP stained smears (3.03) was better than MGG stained smears (2.83). The p-value was highly significant (p=0.003).
Nuclear staining was better in PAP stained smears [39 cases (97.5%) with score 3] than MGG stained smears [36 cases (90%) with score 3]. The p-value was not significant (p=0.07). But, the average score of PAP stained smears (3.03) was better than MGG stained smears (2.9) and the p-value was statistically significant (p=0.017).
Background features were better appreciated in PAP stained smears [40 cases (100%) with score 3] than MGG stained smears [34 cases (85%) with score 3]. The p-value was statistically significant (p=0.011). The average score of PAP stained smears (3.03) was better than MGG stained smears (2.85) and the p-value was significant (p=0.01) [Table/Fig-6].
Effect of staining method on contrast at low power, cytoplasmic features, nuclear features and background features.
| Effect of staining method on contrast at low power |
| Staining method | Number of cases | Average score | p-value* |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| MGG | 0 | 37 | 3 | 0 | 40 | 2.08 | <0.001 (Highly significant) |
| PAP | 0 | 1 | 37 | 2 | 40 | 3.03 |
| Effect of staining method on cytoplasmic features |
| Staining method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| MGG | 0 | 7 | 33 | 0 | 40 | 2.83 | 0.014 (Significant) |
| PAP | 0 | 0 | 39 | 1 | 40 | 3.03 |
| Effect of staining method on nuclear features |
| Staining method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| MGG | 0 | 4 | 36 | 0 | 40 | 2.9 | 0.07(Not significant) |
| PAP | 0 | 0 | 39 | 1 | 40 | 3.03 |
| Effect of staining method on background features |
| Staining method | Number of cases | Average score | p-value |
| Score 1 | Score 2 | Score 3 | Score 4 | Total cases |
| MGG | 0 | 6 | 34 | 0 | 40 | 2.85 | 0.011 (Significant) |
| PAP | 0 | 0 | 40 | 0 | 40 | 3.3 |
*Chi-square test was the statistical tool used to perform the statistical analysis
The average (mean) scores of all the parameters and the overall score were better in PAP stained smears than MGG stained smears. The values were statistically significant [Table/Fig-7].
Comparison of average scores of staining method employed to stain Tzanck smears.
| Parameters | MGG | PAP | p-value* |
|---|
| Mean | Standard deviation | Mean | Standard deviation |
|---|
| Contrast | 2.08 | 0.266 | 3.03 | 0.16 | <0.001 (Highly significant) |
| Cytoplasm | 2.83 | 0.38 | 3.03 | 0.16 | 0.003 (Highly significant) |
| Nucleus | 2.9 | 0.3 | 3.03 | 0.16 | 0.017 (Significant) |
| Background | 2.85 | 0.361 | 3.3 | 0 | 0.01 (Significant) |
| Total score | 10.66 | - | 12.09 | - | - |
*Two sample t-test was the statistical tool used to perform the statistical analysis
Discussion
Although, the skin imparts itself more readily to the cytological examination than any other organ, the cytological diagnosis is much less frequently employed in dermatology than in other fields, like breast pathology, gynaecology (PAP test), bronchopneumology, gastroenterology, urology and endocrinology [4]. In spite of the exponential growth and interest in dermatopathology over many years, and the fact that the skin is the largest desquamating organ in the body, the enthusiasm in cutaneous cytology has been constrained. Although, not a substitute for the histopathology which is considered as the gold standard, Tzanck smears can help in establishing the clinical diagnosis easily and swiftly. It can serve as a useful adjunct to routine histopathology. The technique is inexpensive, easy to perform and does not cause any discomfort to the patient [1,8].
Tzanck smear was introduced as a diagnostic tool for the diagnosis of vesiculobullous lesions especially herpes viral infection. With time, the Tzanck smear has evolved considerably and the cytological finding has been described for several other dermatological conditions like cutaneous infections, immunobullous lesions, genodermatosis, erosives lesions, granulomatous lesions, and cutaneous tumours [8,9].
The total number of cases analysed was highest in the study conducted by Durdu M et al., and least in the study conducted by Prabhala S et al., [10,11]. However, in contrast to other studies, the present study had less number of cases. Yaeen A et al., and Durdu M et al., employed MGG stain to stain the tzanck smears in their study [9,10]. Prabhala S et al., used Giemsa stain [11]. Pawar H et al., employed both Wright-Giemsa stain and PAP stain [8]. In contrast, in the present study, both MGG stain and PAP stain were employed to stain Tzanck smears and were evaluated for staining quality. Similar to the present study, most of the studies showed a wide age range. Durdu M et al., documented a mean age of 36 years in their study [10]. In the present study, clustering of cases was seen in the third decade with mean age of presentation being 31.07 years. Prabhala S et al., documented cutaneous lesions predominantly in males [11]. In contrast, females were predominantly affected in the study conducted by Durdu M et al., and the present study [10]. However, other studies had not commented about the sex predominance in their studies. Yaeen A et al., and the present study documented cutaneous infections as most common cytological diagnosis [9]. In contrast, non-specific inflammatory lesions were common in the study conducted by Pawar H et al., and Prabhala S et al., [8,11] [Table/Fig-8,9].
Comparison of preliminary data in various studies on Tzanck smear cytology [8-11].
| Sl. No. | Authors | No. of cases | Staining methods employed | Age range (years) | Predominant gender | Common diagnosis |
|---|
| 1 | Durdu M et al., [10] (Turkey, 2008) | 400 | MGG | 2-68 | Females | Cutaneous infections (74.75%) |
| 2 | Yaeen A et al., [9] (India, 2015) | 142 | MGG | - | - | Cutaneous infections (70.42%) |
| 3 | Prabhala S et al., [11] (India, 2015) | 21 | Geimsa | 3-62 | Males | Non-specific (52.38%) |
| 4 | Pawar H et al., [8] (India, 2017) | 57 | Wright-Giemsa and PAP | 10-80 | - | Non-specific (35.1%) |
| 5 | Present study | 40 | MGG and PAP | 1 day old new born- 82 | Females | Cutaneous infections (45%) |
Comparison of pattern of distribution of cytological diagnoses on Tzanck smears [8,9,11].
| Sl. No. | Authors | No. of cases | Cutaneous infections | Acute inflammation | Chronic inflammation | Non-specific inflammation | Immuno-bullous lesion | Tumours | Spongiotic dermatitis | Genodermatosis | Non-diagnostic |
|---|
| 1 | Yaeen A et al., [9] (India, 2015) | 142 | 100 (70.42%) | - | - | - | 40 (28.17%) | - | 1 (0.7%) | 1 (0.7%) | - |
| 2 | Prabhala S et al., [11] (India, 2015) | 21 | 8 (38.1%) | - | - | 11 (52.38%) | 2 (9.52) | - | - | - | - |
| 3 | Pawar H et al., [8] (India, 2017) | 57 | 13 (22.81%) | 15 (26.32%) | - | 20 (35.1%) | 9 (15.79%) | - | - | - | - |
| 4 | Present study | 40 | 18 (45%) | 7 (17.5%) | 1 (2.5%) | 8 (20%) | 3 (7.5%) | 1 (2.5%) | - | - | 2 (5%) |
Tzanck smear can be stained by a variety of methods, most commonly by Giemsa stain. Quick staining can be done by Haemacolor or Diff-Quik within one minute. Other stains used are H&E, Wright, methylene blue, and toludine blue [1,4,0,6]. Tzanck smears are routinely stained with MCG stain which has been stated as to be better than PAP stain [4]. But, PAP stain is known to yield polychromatic transparent staining with crisp nuclear and cytoplasmic features [5]. Hence, the present study was undertaken to compare various staining characteristics in Tzanck smears stained by different methods (PAP stained smears and MGG stained smears).
May-Grünwald-Giemsa stain is easy to prepare, reduces the effect of poor techniques and increases the cell yield. But it may produce high background staining obscuring the background and cellular details. Therefore, MGG needs to be prepared freshly every day. MGG stain has tendency to precipitate and depends on technique sensitivity [13]. The PAP stain uses two dyes that differ in their affinity and there is no chemical interaction between nuclear and cytoplasmic solutions as they bind electrostatically, to form salt. PAP has benefit of staining cells from various layers differently [14]. But alcohol fixation may produce distortions and shrinkage unless used at a low temperature [1].
Bhattacharya M et al., conducted a study on oral exfoliative cytology using a scoring system to evaluate the staining characteristics of various stains and concluded than PAP stain proved to be better than H&E, MGG and Leishman-Giemsa Cocktail (LG-Cocktail) [14]. Belgaimi UI and Shetty P suggested that PAP stain and LG-Cocktail were almost identical and superior to MGG [15].
In the present study, scoring system was employed to evaluate the staining characteristic, PAP stained smears were found to be better than MGG stained smears for appreciating contrast, cytoplasm features and background features. The values were found to be statistically significant. Although, the PAP stained smears showed better score than MGG stained smears for appreciating nuclear features, the values were not statistically significant. This was because Chi-square test was used to find out the statistical significance. However, when two sample t-test was employed to find out the statistical significance, the values were found to be significant for appreciating nuclear features. Hence the statistical significance depends on the statistical method employed for analysing the data.
The mean score of all the parameters and overall performance was better in PAP stained smears than MGG stained smears. The values were statistically significant. The earlier literature stated that MGG proves to be better than PAP stain in cutaneous diagnosis [4]. But, the observations in the present study are contradictory to the statement given in the previous literature.
A pathologist has to relay on his observations to judge the staining characteristics and train his eyes to appreciate different cytomorphological features in different stains. Hence, it may be suggested that even though the PAP stained smears scored better than MGG statistically, both the stains may be used as complementary to each other. The disadvantage of one stain may be overcome by the other stain.
Limitation(s)
The number of cases was relatively less in comparison with other studies. Histopathology could be performed in only three cases. This is because in most of the instances, the treatment can be instituted based on the cytological diagnosis. But, in all the three cases, cytological diagnosis was concordant with histopathological diagnosis.
Conclusion(s)
Papanicolaou stain may be considered as a behooveful stain for the evaluation of Tzanck smear. It may be suggested that, although the PAP stained smears scored better than MGG statistically, both the stains may be used as complementary to each other. Each staining method has both pros and cons. The disadvantage of one stain may be overcome by the other stain. A pathologist has to relay on his observations to judge the staining characteristics and train his eyes to appreciate different cytomorphological features in different stains.