Reconsideration of ‘neglected’ antibacterial drugs is one of the approaches for facing this complicated burden of disease as older drugs like temocillin, mecillinam, fusidic acid, polymyxins etc., have documented as potentially useful against MDR pathogens [6,7]. One such agent, fosfomycin, is being called back into play in the United Kingdom (UK) for treating UTI [8,9].
Fosfomycin trometamol is a well-tolerated drug as well as have a broad spectrum of activity against a wide range of Gram-positive and Gram-negative bacteria [10]. It has minimal toxicity, and acts as a time-dependent inhibitor of the MurA enzyme, which catalyses the first committed step of peptidoglycan synthesis involving phosphoenolpyruvate synthetase. As there are no data available on the susceptibility pattern as well as MIC of fosfomycin from this part of country. Hence, the present study was conducted to assess the fosfomycin susceptibility pattern along with MIC against uropathogens by agar dilution method.
Materials and Methods
The present cross-sectional study was conducted in the Department of Microbiology, Government Medical College Haldwani, Uttarakhand, India, between August 2017 to September 2019. Written permission was obtained from Institutional Human Ethical Committee (Letter No. 394/GMC/IEC/2017/Reg. No. 363/IEC/R-16-09-2017). Written informed consent form were signed and collected from the volunteer patients.
Patients visiting the Out-Patient Department (OPDs) with suspected UTIs (symptoms such dysuria with irritating voiding, urinary urgency, frequency, nocturia, painful voiding, pain after voiding, sensation of bladder fullness or lower abdominal discomfort and sometime pain in the suprapubic area) and ≥16 years of age disregarding their gender, were enrolled in the study. All the younger patients <16 years of age and the hospital admitted patient were excluded from the study. Consecutive, non-duplicate, midstream clean catch urine samples were collected in a sterile urine container from the enrolled patients with sign and symptoms along with clinical diagnosis of UTI.
Samples were transported to Microbiology laboratory and processed without any delay. In case of delay, samples were kept at 4-8°C. Wet mount preparation was made directly from the samples and observed under light microscope [Table/Fig-1]. All urine samples were plated semi-quantitatively on CLED agar and incubated at 37°C for overnight. Any suggestive growth was further tested for Gram’s staining and biochemical identifications as per standard operating procedures of the laboratory. They were further subjected to antibiotic susceptibility testing by disc diffusion method and interpreted as per CLSI guidelines, 2017 [11]. The results of the standard single-disc susceptibility tests with disks containing 200 μg of fosfomycin and 50 μg of glucose-6-phosphate were interpreted according to CLSI 2017 guidelines. The zone size ≥16 mm was reported Susceptible (S), while 13-15 mm as Intermediate (I) and ≤12 mm as Resistant (R).
A photograph showing pus cells and red blood cells in urine wet mount preparation.
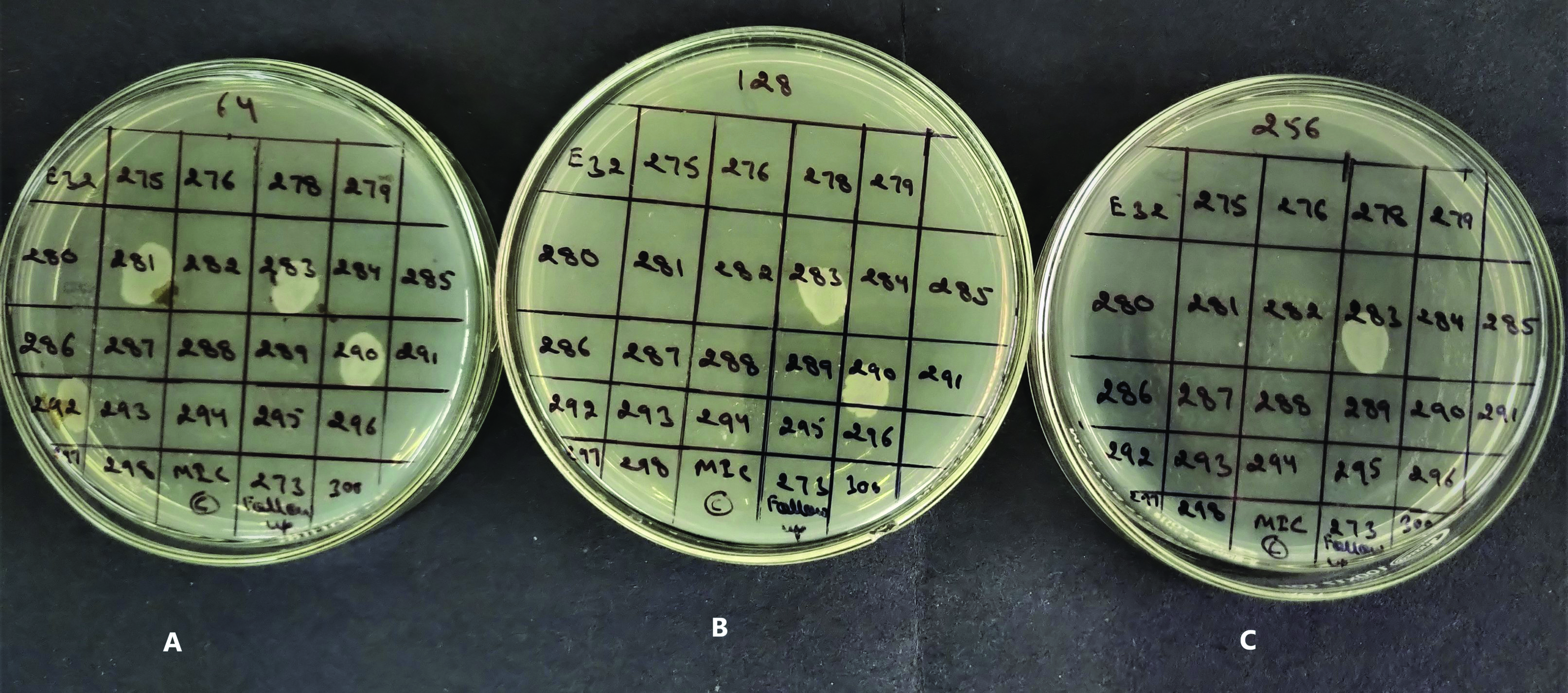
Agar dilution method: The isolates were subjected to MIC testing against fosfomycin trometamol by agar dilution method on Muller Hinton Agar (MHA) supplemented with 25 μg/mL of glucose-6-phosphate to reduce the rate of false resistance as per CLSI guidelines 2017 [11]. Fosfomycin trometamol was used as fosirol powder (Cipla Ltd.,). Muller Hinton Agar with different concentrations of fosfomycin (2, 4, 8, 16, 32, 64, 128, 256 μg/mL) was used. After adjusting the turbidity with 0.5 McFarland standards, 10 μL of bacterial culture of test organism was spot inoculated on MHA plate with different concentrations of fosfomycin. Plates were incubated overnight at 37°C and examined for growth. The MIC values obtained were interpreted according to the following criteria- Susceptible (S) ≤64 μg/mL, Intermediate (I)-128 μg/mL, Resistant (R) ≥256 μg/mL and E. coli ATCC 25922 and E. faecalis ATCC 51299 was used as a control strain (MIC 0.5-2 μg/mL).
Isolates not growing in A (first agar plate) have MIC ≤64 μg/mL, so interpreted as Susceptible. Isolates growing in A but not growing on B (281 and 292) have MIC ≤128 μg/mL, interpreted as intermediate. Isolates growing on B and C are interpreted as resistant having MIC (≥256 μg/mL) [Table/Fig-2].
A photograph showing MIC of fosfomycin against different urinary isolates by agar dilution method.
A=Agar containing fosfomycin trometamol concentration of 64 μg/mL.
B=Agar containing fosfomycin trometamol concentration of 128 μg/mL
C=Agar containing fosfomycin trometamol concentration of 256 μg/mL
Statistical Analysis
Fisher’s-exact test was performed for statistical analysis of the data obtained in the study by using SPSS version 20.0 (IBM SPSS statistics 20). A p-value <0.05 was considered statistically significant.
Results
A total of 2,725 urine samples were tested, out of which 365 had significant growth of urinary bacterial pathogens. The male:female ratio was recorded 04:1 [Table/Fig-3]. Among 365 isolates E. coli dominated the list with 72.32% followed by Enterococcus species (10.41%) [Table/Fig-4].
Age and gender distribution of patients.
| Age range (years) | No. of patients |
|---|
| Male | Female | Total |
|---|
| 16-30 | 31 | 103 | 134 |
| 31-45 | 24 | 65 | 89 |
| 46-60 | 24 | 54 | 78 |
| >60 | 40 | 24 | 64 |
Organism isolated from UTI patients.
| Organisms | Total No. (n=365) | Percentage |
|---|
| E. coli | 264 | 72.32% |
| Enterococcus spp. | 38 | 10.41% |
| Klebsiella spp. | 29 | 7.95% |
| Proteus spp. | 11 | 3.01% |
| Citrobacter spp. | 07 | 1.92% |
| Enterobacter spp. | 07 | 1.92% |
| Pseudomonas spp. | 07 | 1.92% |
| Acinetobacter spp. | 02 | 0.55% |
The antibiotic susceptibility pattern by disc diffusion showed high resistance against cefazolin and trimethoprim/sulphamethoxazole (COT) by all the pathogens except Klebsiella spp. and Citrobacter spp. The resistance against fluroquinolones was also seen in most pathogens except Pseudomonas spp, Enterobacter spp and Proteus spp, E. coli also revealed high rate of resistance towards fluoroquinolones in comparison to other pathogens. Nitrofurantoin was observed very active against members of Enterobacteriaceae family except Proteus spp., which is inherently resistance. Non fermenters also revealed resistance to nitrofurantoin. Fosfomycin was recorded as most sensitive antibiotic against all the pathogens except some species of Proteus [Table/Fig-5].
Organism-wise antibiotic sensitivity pattern by Kirby-Bauer disc-diffusion method among gram-negative uropathogens (n=327).
| Organisms (n=327) | FOS | NIT | COT | CZ | FQ |
|---|
| S | R | S | R | S | R | S | R | S | R |
|---|
| E. coli (n=264) | 260 | 04 | 250 | 14 | 90 | 174 | 77 | 187 | 76 | 188 |
| Klebsiella spp. (n=29) | 26 | 03 | 20 | 09 | 16 | 13 | 15 | 14 | 15 | 14 |
| Proteus spp. (n=11) | 05 | 06 | *NA | *NA | 02 | 09 | 02 | 09 | 07 | 04 |
| Citrobacter spp. (n=07) | 07 | 00 | 06 | 01 | 05 | 02 | 04 | 03 | 04 | 03 |
| Enterobacter spp. (n=07) | 07 | 00 | 05 | 02 | 03 | 04 | 01 | 06 | 05 | 02 |
| Pseudomonas spp. (n=07) | 06 | 01 | *NA | *NA | 02 | 05 | 02 | 05 | 05 | 02 |
| Acinetobacter spp. (n=02) | 02 | 00 | 00 | 02 | 02 | 00 | 00 | 02 | 01 | 01 |
| Total | 313 | 14 | 281 | 28 | 120 | 207 | 101 | 226 | 113 | 214 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
FOS: Fosfomycin; NIT: Nitrofurantoin; COT: Trimethoprim/Sulphamethoxazole; CZ: Cefazolin; FQ: Fluoroquinolones. S: Sensitive, R: Resistant
*NA: Not applied
The Fisher-Exact test was performed for comparing proportions
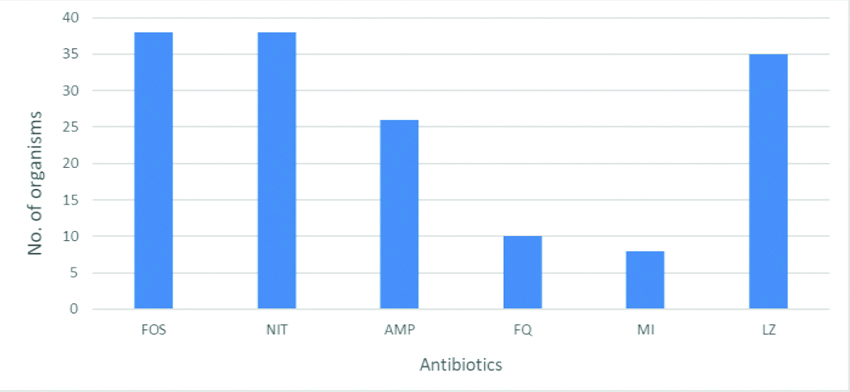
High rates of resistance were seen in Enterococcus spp. against Minocycline and Fluoroquinolones. Fosfomycin and Nitrofurantoin were 100% susceptible to Enterococcus spp. Linezolid and ampicillin also recorded as invitro active antibiotics against Enterococcal isolates [Table/Fig-6].
Antimicrobial sensitivity pattern of enterococcal urinary isolates (n=38)
FOS: Fosfomycin; NIT: Nitrofurantoin; AMP: Ampicillin; FQ: Fluoroquinolones; MI: Minocycline; LZ: Linezolid
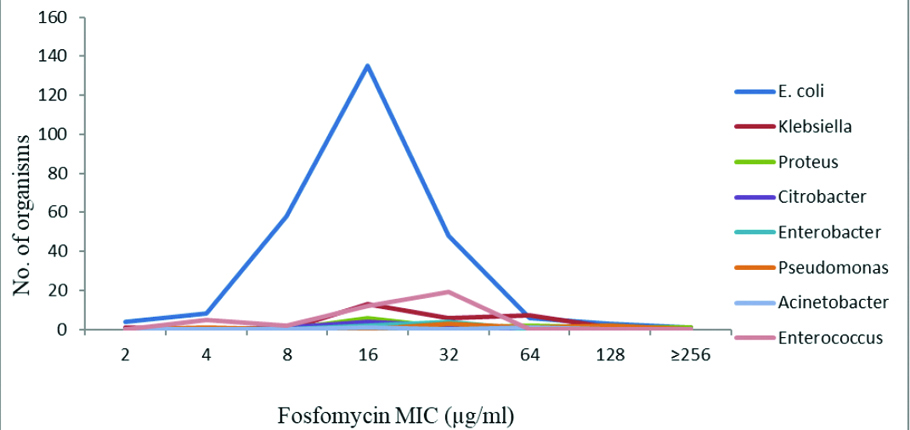
The MIC value of fosfomycin against most of the susceptible uropathogens was noted between 4-64 μg/mL [Table/Fig-7].
Distribution of fosfomycin trometamol MIC by agar dilution method among uropathogens.
Discussion
The UTIs are one of the most common bacterial infections and second most common infectious disease in community and hospitals. UTIs are the most prevailing ailment affecting almost all age groups and both genders. In the present study female were found to be predominant over males among UTI patients except in geriatrics age group (i.e., >60 years). Among the 365 urinary isolates of the current study, majority were gram negative bacilli (89.58%) mainly consisting members of Enterobacteriaceae (97.24%). In the present study E. coli was found to be the predominant pathogen. The result was in concordance with other studies where E. coli were accounted as 70-80% of total urinary isolates [12,13]. There are multiple factors for E. coli being the most common among uropathogens such as it being as most common enteric flora and virulence factors adhesins operative through type-I fimbriae and P fimbriae which helps it to gain entry into urethra [14].
Enterococcus spp. was reported as second most common urinary pathogens in the present study similarly reported by few other studies [15-17]. Enterococcus spp. has been reported as important urinary pathogen in patients with urinary tract abnormalities and related complications [18,19]. The prevalence of Klebsiella spp. in the present study was (7.95%). Similar percentage of Klebsiella spp. were reported by others [1,17,18,20,21]. The prevalence of nonfermenters was recorded (2.75%). Similar results have been reported by few authors previously [1,22].
The treatment of UTIs varies according to the age of the patient, sex, underlying disease, infecting agent and whether there is lower or upper urinary tract involvement. Antibiotics used in the therapy of UTI are usually able to reach high urinary concentrations, which are likely to be clinically effective [23].
As per the Infectious Diseases Society of America (IDSA) guidelines cotrimoxazole is the recommended drug for the treatment of UTIs in settings where the prevalence of resistance is <10-20% and ciprofloxacin is recommended where this resistance is >20%. Co-trimoxazole was preferred as initial first line drug for treatment of UTI [24]. Moreover, Enterococcus species which is second most common gram-positive bacteria causing UTI is inherently resistant to co-trimoxazole [11].
The other agents used in the treatment of UTI include fluoroquinolones, cephalosporins, other β-lactams with or without β-lactamase inhibitors and nitrofurantoin. Recently, several studies have revealed increasing trends of resistance to many antimicrobials including the fluoroquinolones [25-28].
In the present study, 66% of E. coli was reported to be resistant towards Trimethoprim/sulfamethoxazole. Highest resistance was recorded against Proteus spp. (81.82%) and Pseudomonas spp. (71.43%). Acinetobacter spp. was noted as the most sensitive pathogen towards cotrimoxazole. Higher resistance rates were also reported by other authors [25,26]. However, compared to mentioned studies, Sotto A et al., have found low level (26.9%) of resistance to Trimethoprim/sulfamethoxazole [29]. The low cost, widespread availability and usage have led to increase in the resistance of Gram-negative bacilli and also have a disadvantage as Enterococcus spp are inherently resistance Trimethoprim/sulfamethoxazole [11].
In the present study, (71.22%) of all E. coli were found to be resistant to fluoroquinolones. Ciprofloxacin resistance was comparatively less among Gram-negative uropathogens like Pseudomonas spp., Acinetobacter spp., Enterobacter spp. Similar results were observed by Mandal J et al. and Jain P et al., [27,28]. Fluoroquinolones resistance rate was observed 73% in Enterococcus spp. Similar resistance pattern was reported by Mandal J et al., in their study [27].
In the present study, resistance to nitrofurantoin has been observed in E. coli, and Enterococcus spp. i.e., 5.31% and 0%, respectively. Similar resistance pattern has been observed by Manjunath GN et al., and Keepers TR et al., [16,30]. In the present study 31.04%, Klebsiella spp. was found resistant to nitrofurantoin. None of the Acinetobacter spp. was found to be susceptible towards nitrofurantoin. Indeed, despite of its use for long time, nitrofurantoin has retained its broad-spectrum activity against gram-positive and gram-negative bacteria for UTI prophylaxis [21].
In the present study, fosfomycin showed 100% sensitivity in case of Enterococcus spp., Acinetobacter spp., Citrobacter spp. and Enterobacter spp. and resistance in E. coli, Klebsiella spp., Pseudomonas spp were 1.52 %, 10.35% and 14.29%, respectively. There was higher resistance seen in Proteus spp. 54.55% among Enterobacteriaceae family. As compared to present study, Jadoon SA et al., found higher resistance in case of E. coli (5%) and higher susceptibility against Klebsiella spp (100%) [31]. Barry AL and Fuchs PC, have reported 10% of P. aeruginosa strains resistant to fosfomycin while Lu CL et al., have demonstrated higher rates of resistance to fosfomycin among P. aeruginosa isolates [32,33]. The fosfomycin resistance in P. aeruginosa may occur due to over-expression of fosA gene by enzymatic modification of the antibiotic [34].
Out of four, E. coli strains which were interpreted as resistant by disc diffusion method, only one strain was found to have a value of MIC more than 256 μg/mL. A MIC value of 132 μg/mL was observed in other three E. coli strains. There were only one strain each of Klebsiella spp. and Proteus spp. which were also found to be resistant by agar dilution method. Out of 327 gram-negative uropathogens, 298 (91.13%) strains have a MIC value under 32 μg/mL. Among 264 E. coli strains, 205 (77.65%) had a MIC value under MIC 16 μg/mL. All the Enterococcus spp. were found to have a MIC value under 32μg/mL [Table/Fig-7]. The MIC distribution of fosfomycin trometamol in Acinetobacter spp. were 16-64 μg/mL in present study while previous studies show higher MIC breakpoints [33]. Even though fosfomycin was seen sensitive in disc diffusion test and very low MIC breakpoint towards Acinetobacter spp., it remains intrinsically resistant to fosfomycin [11].
Fosfomycin MIC determination study by agar dilution method performed in Kolkata, West Bengal has reported more than 95% susceptibility among Enterobacteriaceae and Enterococcus spp., 73.33% against Pseudomonas spp. while only 50% against Acinetobacter spp. [35]. Another similar study performed by same method at Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry has published 100% Fosfomycin susceptibility in E. coli (0/217), 94.23% in Klebsiella spp. (3/52), 64% in Enterobacter spp. (9/25) and 71.88% in Pseudomonas spp. (9/32) in their reports [36]. In current study, 96.96% (287/316) of Enterobacteriaceae were reported sensitive by agar dilution method which is similar to both studies while all the Enterococcus spp. was found to be susceptible to fosfomycin in present study. One Pseudomonas spp. (14.29%) was reported resistant to fosfomycin in current study while other studies reported only 70-74% susceptibility in their studies. Overall, the resistance to fosfomycin in present study region is comparatively lower than other parts of the country [35,36].
The increasing resistance against fluoroquinolones and co-trimoxazole, drugs which are used as empirical therapy, recommends them not be used for empirical treatment. Resistance against nitrofurantoin is uncommon and is suitable for treatment of uncomplicated lower UTIs.
Limitation(s)
In the present study, genetic identification techniques were not implemented. Molecular screening of resistant isolates is essential to prevent the spread of plasmid- borne resistance against fosfomycin, as the mobility gene may accelerate the dissemination of fosfomycin resistance.
Conclusion(s)
High susceptibility and low MIC distribution of fosfomycin trometamol suggest it along with nitrofurantoin to be used for empirical therapy armamentarium in UTIs among patient visiting day care facilities.
FOS: Fosfomycin; NIT: Nitrofurantoin; COT: Trimethoprim/Sulphamethoxazole; CZ: Cefazolin; FQ: Fluoroquinolones. S: Sensitive, R: Resistant
*NA: Not applied
The Fisher-Exact test was performed for comparing proportions