Evaluation of Change in Aceclofenac Usage Pattern in Knee Osteoarthritis Following Viscosupplementation: A Prospective Interventional Study
Banoth Kiran Kumar1, RK Wadhwa2, Suman Badhal3, C Chethan4, Vinay Kanaujia5, Ajay Gupta6
1 Senior Resident, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, New Delhi, India.
2 Professor and Head, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, New Delhi, India.
3 Professor, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, New Delhi, India.
4 Professor, Department of Physical Medicine and Rehabilitation, AIIMS, Bhopal, Madhya Pradesh, India.
5 Assistant Professor, Department of Physical Medicine and Rehabilitation, SMMH Medical College, Saharanpur, Uttar Pradesh, India.
6 Professor, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ajay Gupta, Room No. 48, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjung Hospital, New Delhi, India.
E-mail: dragupta2013@gmail.com
Introduction
Osteoarthritis (OA) of knee is one of the most common musculoskeletal disorders affecting the elderly population in Asia-Pacific region. Array of diverse treatment options exist including analgesics, Non Steroidal Anti-Inflammatory Drugs (NSAIDs), opioids, physical therapy, orthotic devices, structure modifying drugs, Intra-articular viscosupplementation, corticosteroids or Platelet Rich Plasma (PRP) and surgery. Viscosupplementation {Hyaluronic Acid (HA)} is said to exert an anti-inflammatory effect and has remained a modality under investigation for a longtime.
Aim
To evaluate the change in aceclofenac usage pattern in knee OA following viscosupplementation as a surrogate for efficacy of viscosupplementation related pain relief.
Materials and Methods
This study was a prospective interventional study on 60 subjects over duration of 18 months (October 2015 to March 2017). The subjects who were prescribed viscosupplementation (single dose of Intra-articular Hyaluronic Acid (IAHA) High Molecular Weight (HMW) 90 mg/3 mL in the affected knee) were included after satisfying inclusion and exclusion criteria. All the patients were assessed at the baseline, 4, 8 and 12 weeks in terms of Visual Analogue Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and quantity of aceclofenac intake post viscosupplementation. Data were entered and analysed in Statistical Package of the Social Science (SPSS) version 21. Categorical variables were presented in number and percentage while continuous variables were presented as mean±SD and compared using paired t-test across follow-ups. A p-value ≤0.05 was considered statistically significant.
Results
Total 60 subjects were enrolled in the study, of which 38 (63.33%) were females and 22 (36.67%) were males. After viscosupplementation there was significant reduction in aceclofenac intake from 3.88±1.46 gm to 1.72±0.75 gm and p-value was <0.0001. There was also improvement in pain as VAS decreased from 6.88±0.98 to 3.97±0.86 (p-value <0.0001) over 12 weeks. Similarly there was functional improvement as WOMAC total score reduced from 46.2±8.45 to 27.53±5.67 after 12 weeks. The p-value was <0.0001 at all the follow-ups.
Conclusion
Aceclofenac requirement is decreased and there is improvement in pain and function after viscosupplementation. Viscosupplementation reduces NSAIDs (aceclofenac) usage in OA knee while at the same time reducing pain and improving function.
Knee pain, Visual analogue scale, Western ontario and macmaster universities osteoarthritis index
Introduction
The Osteoarthritis (OA) of the knee is a chronic and progressive disorder, characterised by loss of articular and meniscal cartilage, bone hypertrophy, synovial changes and reduction in the elastoviscosity of the synovial fluid in the joint [1]. According to American College of Rheumatology (ACR) non pharmacological treatment options include patient education, exercises, weight loss, and assistive devices like cane, knee braces and physical modalities. Pharmacological options include topical NSAIDs, oral NSAIDs, glucosamine, chondroitin sulphate, IAHA, corticosteroids and Platelet Rich Plasma (PRP) injections [2]. Surgical treatment options include arthroscopic debridement, osteotomies to redistribute load and total joint replacements, usually explored only when conservative measures fails [3].
Intra-articular viscosupplementation with HA injections has been approved by the US Food and Drug Administration (FDA) exclusively for use in treating pain associated with OA knee in the United States since 1997 [4]. Since HA is a natural component of synovial fluids, HA injections replace this substance and restore the protective effect of healthy synovial fluid by increasing the viscosity of synovial fluid in knee joints affected by OA and potentially reducing and/or counteracting the effects of inflammatory mediators [5].
There are a lot of controversies regarding the clinical use of Intra-articular viscosupplementation for OA knee, but still commonly used as a conservative treatment option over worldwide. American Academy of Orthopedic Surgeons (AAOS) treatment guidelines advised against the use of HA injections for the treatment of the knee OA [6]. ACR have conditional recommendation regarding the viscosupplementation but a position paper issued by them recommends that HA may be considered as a treatment option for OA of the knee [7]. The European League Against Rheumatism (EULAR) recommends that intra-articular HA probably effective in the knee OA [8]. OARSI (OA Research Society International) recommendation for viscosupplementation in the knee OA is uncertain [9].
NSAIDs are being widely used all over the world in OA for their anti-inflammatory and analgesic properties. Aceclofenac from the phenyl acetic acid group of NSAIDs, is a potent blocker of COX-II. It inhibits prostaglandin E2 synthesis. Aceclofenac improves the OA symptoms for a short time interval but it’s prolong use causes heart burn, vertigo, hepatic toxicity, epigastric discomfort, dyspepsia, and abdominal pain [10]. So prolong use of aceclofenac in OA knee is questionable treatment option in view of side effect profile.
Internationally researchers have seen benefits of viscosupplementation on NSAID usage but the same has not been corroborated in the Indian population especially because Indians are believed to at high risk for gastrointestinal side-effects [11]. So, the primary aim of the study is to evaluate the efficacy of viscosupplementation by assessing the quantity of aceclofenac intake, and to evaluate pain (VAS) and function (WOMAC) post viscosupplementation.
Materials and Methods
This study was a single center prospective interventional study conducted in Department of Physical Medicine and Rehabilitation of a tertiary care hospital from October 2015 to March 2017. Approval of Institutional Ethics Committee (IEC/VMMC/SJH/OCTOBER/2015) was taken. Written informed consent was taken from participants, and they were assured of confidentiality of the data and their right to participate in the study.
Inclusion criteria: All the patients who were prescribed viscosupplementation (Single dose of IAHA HMW 90 mg/3 mL in the affected knee) in Physical Medicine and Rehabilitation (PMR) Outpatient Department (OPD) by the treating physician formed the base of this study. Of these, the patients who satisfied the America College of Rheumatology (ACR) were included [12] criteria with Kellegren-Lawrence [13] grade two or three and were prescribed oral aceclofenac by the treating physician during the previous one month. The period of one month prior to viscosupplementation is used as the selection criteria to ensure reasonably accurate recall and treatment records on the use of NSAIDs by the subjects.
Exclusion criteria: Those with history of inflammatory joint disorders, bleeding disorders, peptic-ulcer disease, gastro intestinal bleeding and perforation, pregnancy, diabetes mellitus, hepatic dysfunction and renal dysfunction were excluded.
The study comprised a collection of data on Visual Analogue Scale (VAS) 100 mm, Western Ontario and Macmaster Universities Osteoarthritis Index (WOMAC) (Modified-CRD Pune Version) [14] and quantity of aceclofenac (total dose taken in milligram in a day x number of days) for the period one month prior to viscosupplementation and at 4, 8 and 12 weeks post viscosupplementation. For the purpose of NSAIDs (aceclofenac) usage, patient interview was used for previous data and patient maintained register was used during subsequent follow-ups.
Sample size calculation: Sample size of 60 was calculated using the formula ME=z*(p(1-p))/N) Where z is value of z at two sided alpha error of 5%, N is sample size and ME is margin of error at 5% and p is prevalence rate and taking the estimated prevalence of 3.28% in Delhi population [15].
Statistical Analysis
Data was entered and analysed in Statistical Package for the Social Sciences (SPSS) version 21. Categorical variables like gender and Kellgren and Lawrence (KL) grading were presented in number and percentage while continuous variables like age, VAS score, WOMAC score and quantity of aceclofenac were presented as mean±SD and were compared using paired t-test across follow-ups. A p-value of ≤0.05 was considered statistically significant.
Results
Sixty subjects were enrolled in the study of which 38 (63.33%) were females and 22 (36.67%) were males. Mean age of study population was found to be 62.05±7.97 years. Among 60 subjects included, 17 (28.33%) of grade two and 43 (71.67%) were grade three [Table/Fig-1].
Baseline characteristics of patients (N=60).
| Characteristics | No. of subjects | Percentage |
|---|
| Age group (years) | 41-50 | 6 | 10 |
| 51-60 | 17 | 28.33 |
| 61-70 | 28 | 46.67 |
| 71-80 | 9 | 15 |
| Gender | Female | 38 | 63.33 |
| Male | 22 | 36.67 |
| Kellegren and Lawrence grade | 2 | 17 | 28.33 |
| 3 | 43 | 71.67 |
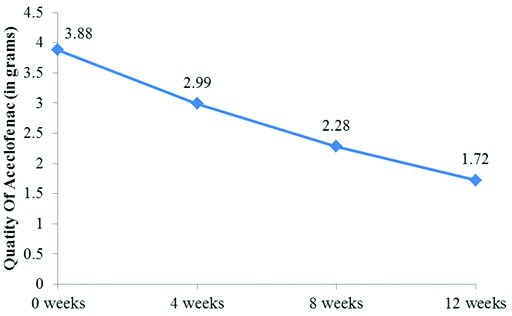
At baseline the range of quantity of aceclofenac was 3.88±1.46 gm during the period of one month prior to viscosupplementation. This significantly reduced on further follow-up at 4, 8 and 12 weeks after viscosupplementation (2.99±1.21 gm, 2.28±1.01 gm and 1.72±0.75 gm). The p-value on comparing baseline to 4, 8 and 12 weeks was <0.0001 [Table/Fig-2,3].
Change in Quantity of aceclofencac intake, VAS Score, WOMAC Score after viscosupplementation and its comparison from baseline.
| Variables | | Baseline | 4 weeks | 8 weeks | 12 weeks |
|---|
| Quantity of Aceclo-fenac (grams) | Mean±SD | 3.88±1.46 | 2.99±1.21 | 2.28±1.01 | 1.72±0.75 |
| p-value compared from baseline | | <0.0001 | <0.0001 | <0.0001 |
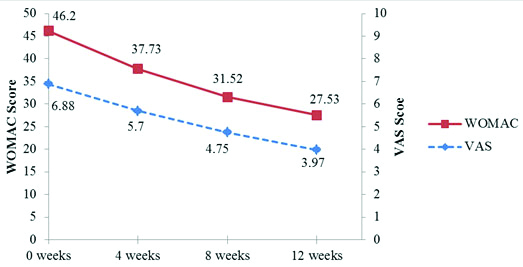
| VAS score | Mean±SD | 6.88±0.98 | 5.7±0.98 | 4.75±1 | 3.97±0.86 |
| p-value compared from baseline | | <0.0001 | <0.0001 | <0.0001 |
| WOMAC score | Mean±SD | 46.2±8.45 | 37.73±7.54 | 31.52±6.53 | 27.53±5.67 |
| p-value compared from baseline | | <0.0001 | <0.0001 | <0.0001 |
Paired t-test was used
Graph showing changes in quantity of Aceclofenac usage after viscosupplementation over consecutive weeks.
At baseline the range of VAS was 6.88±0.98 and WOMAC total was 46.2±8.45 which significantly reduces on further follow-up at 4, 8 and 12 weeks (5.7±0.98, 4.75±1 and 3.97±0.86) and (37.73±7.54, 31.52±6.53 and 27.53±5.67) simultaneously. The p-value on comparing baseline to 4, 8 and 12 weeks was <0.0001 [Table/Fig-2,4].
Graph showing changes in VAS and WOMAC after viscosupplementation over time.
Discussion
The Hyaluronic Acid (HA) forms the backbone of the proteoglycan aggregates necessary for the functional integrity of articular cartilage and other extracellular matrices. HA is believed to have beneficial role for joints which includes an anti-inflammatory effect, acting as lubricant, shock absorbing and energy storing agents between opposing cartilages. HA forms a semi-permeable barrier regulating metabolic exchange between the cartilage and the synovial fluid [16]. Injecting viscosupplementation as local therapy can be beneficial in avoiding many systemic side-effects over NSAIDs such as upper gastro-intestinal bleeding and thereby promises to be a better choice in the management of OA knee.
In present study, there was a significant reduction in aceclofenac intake (p-value <0.0001) after viscosupplementation till 12 weeks. Raynauld JP et al., conducted a randomised controlled, multicentre trial on 255 patients with OA knee to evaluate the efficacy of viscosupplementation [17]. They found that patients receiving HA injections had more improvement in terms of WOMAC pain scale (p-value=0.0001) and reduction in NSAID’s usage (p-value=0.0062) as compared to those patients who did not receive HA injections after one year follow-up. They have not considered any single drug of NSAIDs group in their study but in present study only aceclofenac was taken and compared its quantity of the intake before and after viscosupplementation. The subjects were allowed only aceclofenac in order to keep the data comparable and remove any variations based on the efficacy of different NSAIDs.
In the present study, a significant improvement in pain in term of VAS score after viscusupplementation at 4, 8 and 12 weeks was seen. In a randomised control trial conducted by Huskisson EC and Donnelly S in 100 patients with mild to moderate OA knee with a six month follow-up period, comparing five weekly injection of HA with placebo showed a significant difference for pain in term of VAS at five weeks and this effect was maintained till six months [18]. Similarly other studies [19,20] have also shown significant improvement in pain in term of VAS score. It is also evident from the results that even though when the baseline consumption of NSAIDs was much higher, the relief in pain was suboptimal as seen by significant reduction in VAS scores.
Study conducted by Singhal A et al., on 39 patients of OA knee showed significant improvement in pain and functional index after three months of single intra-articular injection of HMW HA (Hylan G-20) [19]. In present study, authors found significant improvement in both pain and functional subscores of WOMAC. Chevalier X et al., in a randomised controlled, multicentre trial on 253 patients to compare efficacy and safety of a single injection of hylan G-F 20, a HMW HA, versus a placebo has found a statistically significant improvement in WOMAC A (pain) over 4, 8, 12, 18 and 26 weeks post-injection, but had failed to find any significant change in WOMAC C (function) scores versus placebo [21].
Other studies suggest that there is reduction in intake of pain medications after HA injection. Kahan A et al., conducted a randomised study in France population on 506 patients with OA knee to observe the efficacy of HA [22]. They found that patients receiving HA injections had significant improvement in VAS and WOMAC scales and also found a reduction in the NSAID’s intake as compared to those patients who were treated conventionally after 9 months follow-up. Waddell DD and Bricker D conducted a retrospective review over five years on 1047 patients in United States to evaluate the effectiveness and tolerability of HA for treatment of pain in OA knee [23]. They found that pain and mobility was improved and less pain medications was needed after HA as assessed by VAS score, patient related pain and mobility scale and medication scale upto 26 weeks of injection. Viscosupplementation should be considered as a treatment of choice especially for the advantage of reducing the dependence on NSAIDs which have high gastric risk.
Limitation(s)
There is no control group to compare the results. This study has not recorded other confounding factors like physical activity, degenerative joint disorders of other joints, concomitant usage of local therapy and exercises. The study period was limited to 12 weeks and in view of continued improvement at 12 weeks longer follow-up study is recommended.
Conclusion(s)
Nonsurgical treatment options are the cornerstone for treatment of knee OA. The potential benefits of viscosupplementation are recognised by some, but not all, clinical guidelines. From this study it is concluded that viscosupplementation reduces the NSAIDs (aceclofenac) requirement in terms of quantity and also improves the pain and function in patients of knee OA of grades two and three with benefits lasting upto 12 weeks.
Paired t-test was used
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 23, 2020
Manual Googling: Mar 12, 2021
iThenticate Software: Apr 27, 2021 (18%)
[1]. Goldberg VM, Goldberg L, Intra-articular hyaluronans: The treatment of knee pain in osteoarthritisJ Pain Res 2010 3:51-56.10.2147/JPR.S473321197309 [Google Scholar] [CrossRef] [PubMed]
[2]. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and KneeArthritis Care Res (Hoboken) 2020 72(2):149-62.10.1002/acr.2413131908149 [Google Scholar] [CrossRef] [PubMed]
[3]. Adams ME, An analysis of clinical studies of the use of cross-linked hyaluronan, hylan, in the treatment of osteoarthritisJ Rheumatol (suppl) 1993 39:16-18. [Google Scholar]
[4]. Sun S, Chou YJ, Hsu CW, Chen WL, Hyaluronic acid as a treatment for ankle osteoarthritisCurr Rev Musculoskelet Med 2009 2(2):78-82.10.1007/s12178-009-9048-519468874 [Google Scholar] [CrossRef] [PubMed]
[5]. Thomas T, Amouroux F, Vincent P, Intra-articular hyaluronic acid in the management of knee osteoarthritis: Pharmaco-economic study from the perspective of the national health insurance systemPLoS One 2017 12(3):e017368310.1371/journal.pone.017368328328935 [Google Scholar] [CrossRef] [PubMed]
[6]. Jevsevar DS, Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd editionJ Am Acad Orthop Surg 2013 21(9):571-76.10.5435/00124635-201309020-00008 [Google Scholar] [CrossRef]
[7]. American College of Rheumatology. Intra-Articular Hyaluronic Acid Injection in Osteoarthritis of the Knee. Atlanta, GA: American College of Rheumatology; 2012. [Accessed December 1, 2017]. Available from: https://www.rheumatology.org/Portals/0/Files/Viscosupplementation.pdf [Google Scholar]
[8]. Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, EULAR recommendations for the management of knee osteoarthritis: Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)Ann Rheum Dis 2000 59(12):936-44.10.1136/ard.59.12.93611087696 [Google Scholar] [CrossRef] [PubMed]
[9]. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, OARSI guidelines for the non-surgical management of knee osteoarthritisOsteoarthritis Cartilage 2014 22(3):363-88.10.1016/j.joca.2014.01.00324462672 [Google Scholar] [CrossRef] [PubMed]
[10]. Kornasoff D, Maisenbacher J, Bowdler J, Raber A, The efficacy and tolerability of aceclofenac compared to indomethacin in patients with rheumatoid arthritisRheumatol Int 1996 15(6):225-30.10.1007/BF002903758778950 [Google Scholar] [CrossRef] [PubMed]
[11]. Chatterjee S, Dureja GP, Kadhe G, Mane A, Phansalkar AA, Sawant S, Cross-sectional study for prevalence of non-steroidal anti-inflammatory drug-induced gastrointestinal, cardiac and renal complications in India: Interim reportGastroenterology Res 2015 8(3-4):216-21.10.14740/gr658w27785299 [Google Scholar] [CrossRef] [PubMed]
[12]. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism AssociationArthritis Rheum 1986 29(8):1039-49.10.1002/art.17802908163741515 [Google Scholar] [CrossRef] [PubMed]
[13]. Kellegren JH, Lawrence JS, Radiological assessment of osteo-arthrosisAnn Rheum Dis 1957 16(4):494-502.10.1136/ard.16.4.49413498604 [Google Scholar] [CrossRef] [PubMed]
[14]. Chopra A, Rheumatology: Made in India (Camps, COPCORD, HLA, Ayurveda, HAQ, WOMAC and drug trials)J Indian Rheum Assoc 2004 12:43-53. [Google Scholar]
[15]. Sharma R, Epidemiology of Musculoskeletal Conditions in India 2012 New Delhi, IndiaIndian Council of Medical Research (ICMR) [Google Scholar]
[16]. Peyron JG, A new approach to the treatment of osteoarthritis: ViscosupplementationOsteoarthritis Cartilage 1993 1(2):85-87.10.1016/S1063-4584(05)80022-6 [Google Scholar] [CrossRef]
[17]. Raynauld JP, Torrance GW, Band PA, Goldsmith CH, Tugwell P, Walker V, Canadian Knee OA Study GroupA prospective, randomised, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): Clinical resultsOsteoarthritis Cartilage 2002 10(7):506-17.10.1053/joca.2002.079812127830 [Google Scholar] [CrossRef] [PubMed]
[18]. Huskisson EC, Donnelly S, Hyaluronic acid in the treatment of osteoarthritis of the kneeRheumatology (Oxford) 1999 38(7):602-07.10.1093/rheumatology/38.7.60210461471 [Google Scholar] [CrossRef] [PubMed]
[19]. Singhal A, Marwaha V, Hande V, Sud AD, Efficacy and safety of viscosupplementation in symptomatic knee osteoarthritis: An experience from a Tertiary Care Center in MumbaiJ Mar Med Soc 2017 19:24-27.10.4103/jmms.jmms_5_16 [Google Scholar] [CrossRef]
[20]. Kanaujia V, Gupta A, Sharma DK, Verma S, Yadav RK, Study of effectiveness of lateral wedge insole on medial compartment of osteoarthritis of knee treated with viscosupplementationIndian J Pain 2020 34:106-11. [Google Scholar]
[21]. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: A randomised, multicentre, double-blind, placebo controlled trialAnn Rheum Dis 2010 69(1):113-19.10.1136/ard.2008.09462319304567 [Google Scholar] [CrossRef] [PubMed]
[22]. Kahan A, Lleu PL, Salin L, Prospective randomised study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritisJoint Bone Spine 2003 70(4):276-81.10.1016/S1297-319X(03)00043-5 [Google Scholar] [CrossRef]
[23]. Waddell DD, Bricker DC, Clinical experience with the effectiveness and tolerability of hylan G-F 20 in 1047 patients with osteoarthritis of the kneeJ Knee Surg 2006 19(1):19-27.10.1055/s-0030-124807216468490 [Google Scholar] [CrossRef] [PubMed]