Incidental Finding of Distal Ulnar Giant Cell Tumour Treated with Preoperative Short Course of Denosumab and Extended Curettage
Mohit Singh1, Sudip Deb2, Nungdilong3, Pranay Gupta4, Ajay Kumar Yadav5
1 Senior Resident, Department of Orthopaedics, Lady Hardinge Medical College and SSK Hospital, New Delhi, India.
2 Senior Resident, Department of Orthopaedics, Andaman and Nicobar Islands Institute of Medical Sciences, Port Blair, Andaman and Nicobar, India.
3 Senior Resident, Department of Orthopaedics, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India.
4 Senior Resident, Department of Orthopaedics, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India.
5 Senior Resident, Department of Orthopaedics, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Pranay Gupta, Senior Resident, Department of Orthopaedics, Room No. 207, Doctor Hostel, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
E-mail: pranay16gta@gmail.com
Giant Cell Tumour (GCT) forms 5% of all primary bone tumours. GCT of distal ulna is an extremely uncommon entity being more common in the distal femur, proximal tibia and distal radius, respectively. Wide excision is one of the modalities of treatment, but this creates a bony defect that have to be reconstructed. The case report discusses a 30-year-old Indian male who was diagnosed incidentally with GCT of distal ulna after an alleged history of injury following road traffic accident. It was extremely difficult to perform excision and extended curettage initially due to extensive thinning of the cortex. Denosumab was administered subcutaneously for three months. Daily supplements of calcium 500 mg and vitamin D 400 IU were given. The short course of preoperative Denosumab lead to marked intralesional sclerosis facilitating the less morbid procedure of extended curettage and bone grafting and thus, salvaging the wrist. The functional outcome at one year postoperatively was excellent without any complications. Denosumab has the potential to regress the tumour, thereby preventing the need for complex reconstructive surgeries of the wrist.
Bone grafting, Chemical cauterisation, Distal ulna swelling, Neoadjuvant denosumab, Primary bone tumour
Case Report
A 30-year-old male presented to the Outpatient Department with pain and swelling in the left wrist for the last two months. There was a history of trivial trauma and injury to the left wrist following an alleged road traffic accident, two months back. The pain was sudden in onset, continuous in nature, moderate in intensity, aggravated by physical activity and relieved by rest. The patient was initially treated with alternative medicine, which helped reduction of the pain and swelling at that time.
On examination, there was a diffuse swelling over the distal aspect of the left ulna. The condition of the skin over the swelling was normal with no evidence of venous engorgement. There was no scar mark or visible sinuses. On palpation, there was tenderness around swelling without any local rise of temperature. The swelling was approximately 1.5×2 cm in dimension, arising from the distal end of ulna firm in consistency, immobile and not fix to the overlying skin. The active range of motion of left wrist joint was 20° supination, 25° pronation, 30° dorsiflexion, 45° palmar flexion, 15° radial deviation and 10° ulnar deviation. A plain radiograph of the left forearm with wrist anteroposterior and lateral view was performed immediately. The plain X-ray showed an expansile osteolytic lesion with extreme cortical thinning at the distal end of the ulna [Table/Fig-1a]. The articular surface maintained its contiguity with the surrounding normal bone. A magnetic resonance imaging was further advised which confirmed the X-ray finding and showed an expansile lytic locally aggressive space-occupying lesion at the distal metaphyseal end of ulna without periosseous soft tissue invasion [Table/Fig-1b,c].
(a) Plain radiograph of affected left wrist anteroposterior and lateral view showing lytic lesion of the distal ulna; (b, c) MRI of wrist T1 and T2 weighted image suggestive of an expansile lytic locally aggressive space-occupying lesion at the distal metaphyseal end of the ulna; (d) Histopathological slide of core needle biopsy showing multinucleated giant cells in a sea of mononuclear cells on (H&E, 10x); (e) X-ray of affected left wrist after denosumab therapy showing consolidation of the cortex of bone.
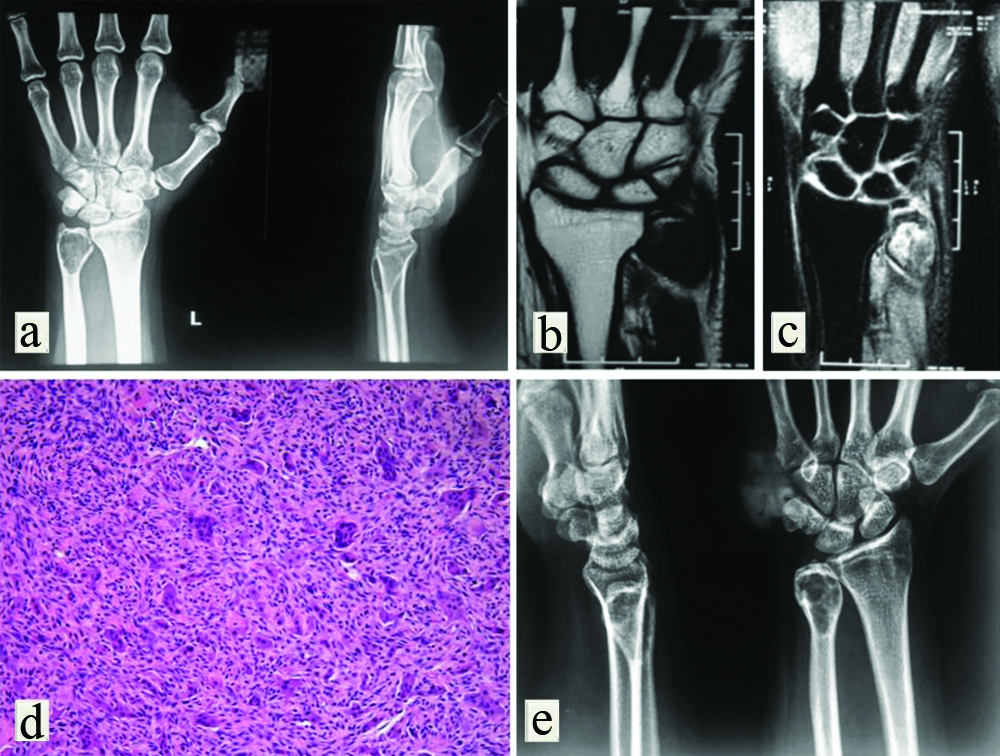
Further to confirm the diagnosis, core needle biopsy was taken from distal ulnar swelling. The report showed cortical bone with plump to spindle shape cell with few multinucleated giant cells and focal new bone formation, suggestive of GCT [Table/Fig-1d]. It was extremely difficult to perform excision and extended curettage initially due to extensive thinning of the cortex. The salvation of the distal end of the ulna and associated wrist joint was very much needed to maintain the patient’s professional accuracy and daily living as a freelance photographer. So, it was planned to administer preoperative short-course therapy with subcutaneous denosumab to achieve intra and perilesional remission. Accordingly, denosumab was administered subcutaneously 120 mg every four weeks with additional loading doses of 120 mg on days 8 and 15 of the first month for a period of three months. Daily supplements of calcium 500 mg and vitamin D 400 IU were given and serum electrolytes were checked every four weeks. Denosumab therapy leads to marked intralesional sclerosis and thickening of the cortex by the perilesional new bone formation of the distal ulna [Table/Fig-1e]. It decreased the tumour progression as well as surgically down-staged the tumour, thereby facilitating extended curettage and bone grafting.
After three months of post denosumab therapy, an extended curettage of the tumour was done with the help of chemical cauterisation (using hydrogen peroxide), thermal cauterisation (using bipolar cautery) along with autologous cancellous bone grafting from the ipsilateral iliac crest [Table/Fig-2a]. After surgery, below elbow Plaster of Paris slab was applied.
(a) Intraoperative picture during the procedure of curettage and removal of GCT followed by bone grafting; (b)X-ray of the wrist after one year showing graft uptake and well-consolidated cortex; (c,d) Images showing wrist movements palmar and dorsiflexion after one year.
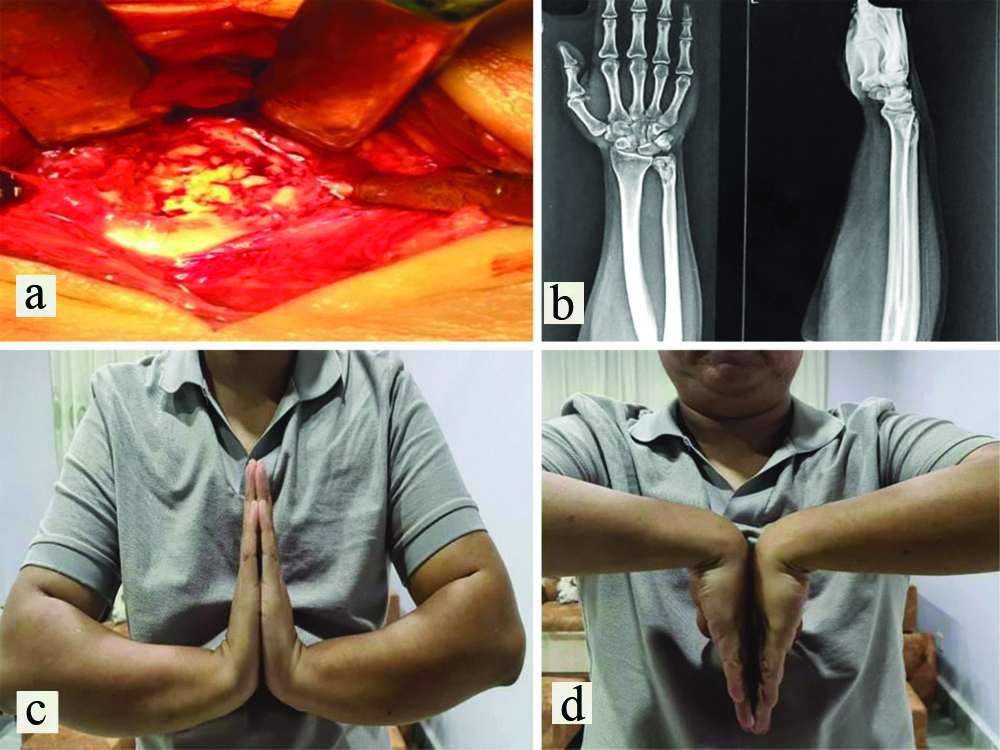
Postoperatively, the patient was comfortable without much complaint except for the surgical site pain. The suture removal was done on 14th postoperative day. Finger movements were encouraged throughout the postoperative period. Active physiotherapy of wrist and finger started from postoperative third week onwards. The patient was followed-up on the third week and sixth week postoperative and once in a month thereafter for a period of one year. The radiograph done after six weeks of surgery demonstrated the sclerotic changes which indicates the presence of bony graft in-situ. The patient’s wrist was pain-free and had acceptable functional outcome at one year postoperatively without any complications and recurrence as seen clinically and radiologically [Table/Fig-2b-d].
Discussion
A GCT is a benign, epiphyseal growing but locally aggressive in nature, accounting for 5% of all primary bone tumour [1]. It is most commonly seen in 20 to 40 years with slightly female predominance. The most common sites are distal femur, proximal tibia and distal radius, respectively [2]. The distal ulna is a very unusual place for GCT, encompassing about 0.45 to 3.2% of all GCT [3]. It usually presents with pain and pathological fractures in about 10 to 30% [4]. Traditionally, GCT has been treated surgically by local curettage, with or without packing of the defect with polymethylmethacrylate (PMMA) cement or bone graft, and internal fixation when needed. Extended curettage is done with the use of adjuvants like liquid nitrogen, phenol, PMMA and argon laser. Treatment of GCT of the distal radius or ulna poses a surgical challenge, as complex reconstructive surgery may be required [5].
Denosumab is a human monoclonal antibody. It is used for the treatment of osteoporosis, bone loss, bone metastases and giant cell tumor of bone. Receptor Activator For Nuclear Factor κ B (RANK) is activated by RANKL (the RANK-Ligand), which exists as cell surface molecules on osteoblasts. Activation of RANK by RANKL promotes the maturation of pre-osteoclasts into osteoclasts. Denosumab inhibits this maturation of osteoclasts by binding to and inhibiting RANKL and protects bone from degradation [6,7].
Juxtra-articular GCT can pose major surgical challenges and sometimes needs en-bloc excision [8]. Aggressive GCT often requires complex reconstructive surgery and has a tendency to recur locally. Denosumab has emerged as a possible medical treatment of GCT as it stops the progression of the GCT, improves pain and increases perilesional bone formation [9].
The index case shows that denosumab treatment before the surgical procedure for a large GCT helps in regression and resection of the tumour or extended curettage without spillage of the tumour cells as also have been seen in studies of Girolami I et al., Lau CP et al., and Mak IW et al., even though it could not be confirmed if the denosumab lowers the recurrence rate [10-12]. Agarwal MG et al., and van Langevelde K and McCarthy CL, used the dose of 120 mg on days 1,8,15 and 29 followed by every four weeks till three months [9,13]. In this case, denosumab was administered subcutaneously 120 mg every four weeks with additional loading doses of 120 mg on days 8 and 15 of the first month for a period of three months. Comprehensive narrative literature of Kumar R et al., and Rutkowski P et al., shows that preoperative denosumab therapy followed by surgery in the management of GCT is associated with clinical, histopathological and therapeutical benefits [14,15]. It is also associated with better tolerability, safety, surgical downstaging, less morbid salvageable procedures and favourable outcomes. The case studies of Chawla S et al., in 2013 and Scoccianti G et al., in 2016 were held on 282 patients and on 97 patients, respectively [16,17]. These studies also favour the use of the denosumab to facilitating curettage instead of osteoarticular resection. So, use of denosumab not only facilitates curettage but also decrease the chances of high complex reconstructive surgeries.
Conclusion(s)
Juxtra-articular GCT of ulna can appear as incidental finding in post-traumatic cases. The neoadjuvant denosumab therapy along with extended curettage and bone grafting helps in preservation of wrist joint functional anatomy.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 16, 2021
Manual Googling: May 06, 2021
iThenticate Software: May 26, 2021 (19%)
[1]. Eckardt JJ, Grogan TJ, Giant cell tumor of boneClin Orthop Relat Res 1986 (204):45-58.10.1097/00003086-198603000-00006 [Google Scholar] [CrossRef]
[2]. Bridge JA, Neff JR, Mouron BJ, Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literatureCancer Genet Cytogenet 1992 58(1):02-13.10.1016/0165-4608(92)90125-R [Google Scholar] [CrossRef]
[3]. Goldenberg RR, Campbell CJ, Bonfiglio M, Giant-cell tumor of bone. An analysis of two hundred and eighteen casesJ Bone Joint Surg Am 1970 52(4):619-64.10.2106/00004623-197052040-000015479455 [Google Scholar] [CrossRef] [PubMed]
[4]. van der Heijden L, Dijkstra PD, Campanacci DA, Gibbons CL, van de Sande MA, Giant cell tumor with pathologic fracture: Should we curette or resect?Clin Orthop Relat Res 2013 471(3):820-29.10.1007/s11999-012-2546-622926445 [Google Scholar] [CrossRef] [PubMed]
[5]. Puri A, Agarwal M, Treatment of giant cell tumor of bone: Current conceptsIndian J Orthop 2007 41(2):101-08.10.4103/0019-5413.3203921139760 [Google Scholar] [CrossRef] [PubMed]
[6]. Hanley DA, Adachi JD, Bell A, Brown V, Denosumab: Mechanism of action and clinical outcomesInt J Clin Pract 2012 66(12):1139-46.10.1111/ijcp.1202222967310 [Google Scholar] [CrossRef] [PubMed]
[7]. Xu SF, Adams B, Yu XC, Xu M, Denosumab and giant cell tumour of bone-a review and future management considerationsCurr Oncol 2013 20(5):e442-47.10.3747/co.20.149724155640 [Google Scholar] [CrossRef] [PubMed]
[8]. Hashizume H, Kawai A, Nishida K, Sasaki K, Inoue H, Ulnar buttress arthroplasty for reconstruction after resection of the distal ulna for giant cell tumourJ Hand Surg Br 1996 21(2):213-15.10.1016/S0266-7681(96)80101-X [Google Scholar] [CrossRef]
[9]. Agarwal MG, Gundavda MK, Gupta R, Reddy R, Does Denosumab change the giant cell tumor treatment strategy? Lessons learned from early experienceClin Orthop Relat Res 2018 476(9):1773-82.10.1007/s11999.000000000000024330794215 [Google Scholar] [CrossRef] [PubMed]
[10]. Girolami I, Mancini I, Simoni A, Baldi GG, Simi L, Campanacci D, Denosumab treated giant cell tumour of bone: A morphological, immunohistochemical and molecular analysis of a seriesJ Clin Pathol 2016 69(3):240-47.Epub 2015 Sep 310.1136/jclinpath-2015-20324826338802 [Google Scholar] [CrossRef] [PubMed]
[11]. Lau CP, Huang L, Wong KC, Kumta SM, Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of boneConnect Tissue Res 2013 54(6):439-49.10.3109/03008207.2013.84820224060052 [Google Scholar] [CrossRef] [PubMed]
[12]. Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M, A Translational Study of the Neoplastic Cells of Giant Cell Tumor of Bone Following Neoadjuvant DenosumabJ Bone Joint Surg Am 2014 96(15):e12710.2106/JBJS.M.0133225100780 [Google Scholar] [CrossRef] [PubMed]
[13]. van Langevelde K, McCarthy CL, Radiological findings of denosumab treatment for giant cell tumours of boneSkeletal Radiol 2020 49(9):1345-58.10.1007/s00256-020-03449-132335707 [Google Scholar] [CrossRef] [PubMed]
[14]. Kumar R, Meis JM, Amini B, McEnery KW, Madewell JE, Rhines LD, Giant cell tumor of cervical spine presenting as acute asphyxia: Successful surgical resection after down-staging with DenosumabSpine (Phila Pa 1976) 2017 42(10):E629-32.10.1097/BRS.000000000000195127792106 [Google Scholar] [CrossRef] [PubMed]
[15]. Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of boneAnn Surg Oncol 2015 22(9):2860-68.10.1245/s10434-015-4634-926033180 [Google Scholar] [CrossRef] [PubMed]
[16]. Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 studyLancet Oncol 2013 14(9):901-08.10.1016/S1470-2045(13)70277-8 [Google Scholar] [CrossRef]
[17]. Scoccianti G, Totti F, Scorianz M, Baldi G, Roselli G, Beltrami G, Preoperative Denosumab with curettage and cryotherapy in giant cell tumor of bone: Is there an increased risk of local recurrence?Clin Orthop Relat Res 2018 476(9):1783-90.10.1007/s11999.000000000000010430778015 [Google Scholar] [CrossRef] [PubMed]