Multiple myeloma is characterised by malignant proliferation of plasma cells in bone marrow with rare involvement of peripheral blood (plasma cell leukaemia). Sometimes tissue involvement (plasmacytoma) is also seen. However, pleural effusion is very rare in myeloma patients. Pleural effusion in myeloma is usually secondary and reactive in nature. Malignant Myelomatous Pleural Effusion (MPE) is usually associated with poor prognosis. Hereby, the author report a case of a 46-year-old male who presented with groin pain with ureteric calculus. The patient was diagnosed with multiple myeloma with 42% plasma cells in the marrow. On treatment, patient went into remission, however he relapsed twice. On second relapse, the marrow examination showed plasma cells with plasmablastic morphology (24%). Plasmablastic morphology is associated with poor prognosis. The patient also developed pleural effusion. The cytospin smears of the pleural fluid showed clusters of atypical plasma cells (positive for CD38, CD138 and kappa light chain restriction). Thus, the present case report an extremely rare presentation of multiple myeloma with plasmablastic morphology and MPE.
Case Report
A 46-year-old male presented in the Department of Urology with complaint of groin pain secondary to ureteric calculus. Haemogram showed low haemoglobin (9.2 g/dL), normal leucocyte count (8400 cells/cumm) and low platelet count (76,000/cumm). Peripheral smear showed rouleax formation and thrombocytopenia. Bone marrow examination showed diffuse proliferation of plasma cells (42%). Serum Immunofixation Electrophoresis (IFE) showed Immunoglobin G (IgG) kappa monoclonal gammapathy. He underwent 16 cycles of bortezomib and lenolidomide chemotherapy followed by bone marrow trephine biopsy which showed no detectable plasma cells (remission) and his paraprotein levels reduced significantly to 6.8 g/L.
The patient remained in remission until 2018 when he developed shortness of breath and mild chest pain. On chest X-ray, there was mild pleural effusion on the right side. The fluid was drained and sent for laboratory examination. Biochemical analysis of the pleural fluid showed that it was an exudate (protein was 3.4, Lactate Dehydrogenase (LDH) was 224, glucose-80 mg/dL) and on fluid cytology there was predominance of lymphocytes along with few neutrophils. Culture was negative. However, there was rise in serum IgG paraprotein levels (850 mg/dL). On bone marrow trephine biopsy, there were 56% plasma cells in the marrow, suggestive of relapse. On serum electrophoresis, M band was detected and on serum IFE, band was seen in IgG kappa region. Patient underwent bone marrow transplant, followed by 12 cycles of chemotherapy.
In October 2019, the patient again presented with on and off abdominal pain and constipation for 15 days, high grade fever and breathlessness for two days. On examination, blood pressure was 90/60 mm/Hg, pulse was 80 beats/min, SpO2 was 92%. On chest examination, there was dullness on percussion on the right side.
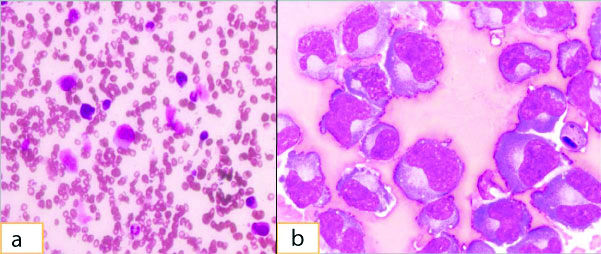
Haemogram showed haemoglobin 8.7 gm/dL, total leucocyte count was 10,000/cumm, neutrophils was 90% and platelet count was 74,000/cumm. Peripheral smear showed rouleax formation, neutrophilia and thrombocytopenia. Serum albumin was 3.0 mg/dL, serum globulin was 2.6 gm/dL, serum b2 microglobulin was 5.6 mg/dL, serum calcium was 8.5 mg/dL, serum creatinine was 5.4 mg/dL, serum LDH was 313 mg/dL and C reactive protein 19.65 mg/L. Bone marrow examination showed plasma cells with plasmablastic morphology (24%) [Table/Fig-1a]. Chest X-ray examination showed right sided mild pleural effusion. The pleural fluid was drained and sent for routine examination, cytology and flow cytometry. The fluid was haemorrhagic and cytospin smears and cell blocks showed singly scattered as well as clusters of atypical plasma cells with eccentrically placed hyperchromatic nuclei, some showing prominent nucleoli [Table/Fig-1b]. Few binucleated plasma cells were also seen.
(a) Histological findings, H&E,100x, Bone Marrow aspirate smears showing plasma cells; (b) Histological findings, H&E,100x, Pleural fluid cytospin smears showing plasma cells with plasmablastic morphology.
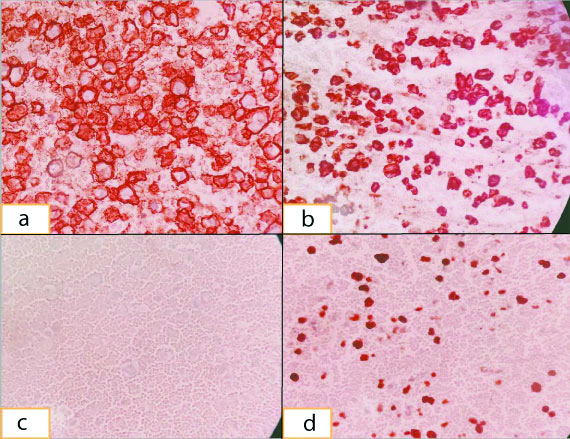
On immunohistochemistry of cell block, cells were positive for CD138 [Table/Fig-2a], CD38, with kappa light chain restriction [Table/Fig-2b], lambda negative [Table/Fig-2c] and high Ki67 [Table/Fig-2d]. Flow cytometry of the pleural fluid showed a cluster of abnormal cells (43%) in SSC/CD45 dot plot with forward scatter falling in the small and intermediate cell size range. This abnormal population showed kappa restriction and was positive for CD38. Cells were negative for Surface Membrane Immunoglobulin (SMIg) lambda, CD56, CD10, CD19 and CD45. So the fluid was reported as positive for malignant cells and suggestive of myelomatous effusion.
(a) Immunohistochemistry, 40X, Plasma cells with CD138 positivity; (b) Immunohistochemistry, 40X, Plasma cells with Kappa light chain positivity; (c) Immunohistochemistry, 40X, Lambda light chain with negative plasma cells; (d) Immunohistochemistry, 40X, Plasma cells with high Ki67 positivity.
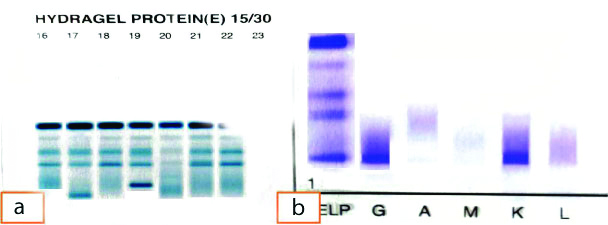
On serum electrophoresis, total protein was 5 g/dL, serum albumin was 3.2 g/dL, globulin was 1.8 g/dL, alpha-1 globulin was 0.19 gm%, alpha-2 globulin was 0.75%, beta globulin was 0.59 gm%, gamma globulin 0.42 gm%. No M band was seen. On IFE, a faint band was seen in kappa region. Free kappa (κ) was 37.69 mg/L, free lambda (λ) was 21.14 mg/L, κ/λ ratio was 1.78. Pleural fluid electrophoresis showed an M band [Table/Fig-3a] and IFE showed IgG kappa light chain monopathy [Table/Fig-3b]. Thus, the patient was diagnosed as relapsed multiple myeloma with MPE. The patient was managed on immunochemotherapy along with antibiotics but he deceived after eight months of treatment.
(a) Protein electrophoresis shows M Band; (b) Immunofixation Electropheresis shows IgG kappa light chain positivity.
Discussion
Plasma cell myeloma comprises about 1% of all malignant tumours and 10-15% of haematopoeitic tumours. It is a bone marrow based multifocal monoclonal plasma cell proliferation and characteristically associated with monoclonal protein in serum and/or urine. It typically shows generalised bone marrow involvement however, extramedullary involvement is seen with advanced stage of disease. Clinical spectrum ranges from asymptomatic to aggressive disease state due to deposition of abnormal proteins in tissue [1]. Myeloma patients commonly present with hypercalcaemia, renal insufficiency, anaemia and/or bony lesions. Only 6% patients of myeloma develop pleural effusion during the course of the disease [2]. Most of the cases of pleural effusion in myeloma patients are secondary to concomitant disease process. However, effusion related to infiltration of the pleura by plasma cells i.e., MPE, are extremely rare accounting for <1% patients of myeloma [3]. Myelomatous Pleural Effusion (MPE) is usually a sign of advanced disease, but very rarely patients presents with pleural effusion as an initial manifestation [4-6].
Most of the cases of pleural effusions in myeloma patients are reactive to non malignant causes, the most common being congestive cardiac failure due to amyloidosis followed by pulmonary embolism, infections, nephrotic syndrome and chronic renal failure [7]. Malignant pleural effusions are mainly due to secondary neoplasms like carcinomas of lung, mesothelioma and carcinoma breast [8]. So, it is very important to investigate pleural effusion in detail and exclude all the malignant and non malignant causes. In this case, patient had normal cardiac functions and there was no evidence of pulmonary embolism or any infective aetiology or any secondary neoplasm. The lack of alternate diagnosis and the presence of atypical plasma cells in the pleural fluid support the diagnosis of MPE in this patient.
In this case, the bone marrow examination showed plasma cells with plasmablastic morphology (24%), the cytospin smears and cell blocks of pleural effusion showed singly scattered as well as clusters of atypical plasma cells with eccentrically placed hyperchromatic nuclei, some showing prominent nucleoli. It is difficult to differentiate reactive plasma cells from clonal plasma cells merely on morphology unless plasma cells are present in gross excess, thereby making a clonal proliferation more likely. Reactive plasma cells can show varied morphology including multinucleate, pleomorphic/atypical plasma cells. Hence, morphology alone cannot be relied for the diagnosis of neoplasia.
Normal plasma cell expresses CD45dim, CD19, CD38 bright and CD138. CD38 and CD138 are also expressed on neoplastic plasma cells, so positivity for these antigens is not discriminatory, but the intensity of expression does change on flow cytometry. CD38 is dimmer and CD138 is brighter in malignant plasma cell population compared to their normal counterpart on flowcytometry. Neoplastic plasma cells are often CD45 negative together with diminished or absent CD19. In cases of myeloma relapse, especially cases with acquired TP53 mutation of the plasma cells, the plasma cells may develop plasmablastic morphology. Their appearance often signifies a near terminal stage of disease [9]. Immature plasma cells contribute to the development of MPEs and explains the aggressive nature of myelomatous disease [10]. In the index case, the pleural cavity was invaded by malignant plasma cells, which was confirmed with cytology, cell block preparation, Immunohistochemistry and flow cytometry of the fluid.
In 1994, diagnostic criteria were defined to diagnose and confirm a MPE: (a) demonstration of a monoclonal protein in pleural fluid electrophoresis; (b) detection of atypical plasma cells in the pleural fluid; and (c) histologic confirmation with a pleural biopsy sample or by autopsy. In the literature, 80% of MPE is associated with IgA monoclonal protein [5,11]. However, in the patient in this case, serum and pleural protein electrophoresis showed IgG kappa-light chain monoclonopathy.
Pleural effusion is usually a late manifestation of myeloma or shows aggressive course of the disease and associated with poor prognosis with survival usually less than four months even when chemotherapy is given [12-15]. Abhishek K et al., reported three patients with multiple myeloma, who presented with different thoracic manifestations such as left-sided MPE, posterior mediastinal mass with central airway obstruction, and pulmonary consolidation and survival period was very less [16]. The index patient had a somewhat longer survival (eight months) after chemotherapy.
Conclusion(s)
Pleural effusion in plasma cell myeloma is rare and when present, it is usually associated with concomitant disease process and is reactive in nature. MPE is extremely rare and associated with aggressive nature of the disease and poor clinical outcome. So, it is very important to carefully examine the fluid specimen for presence of plasma cells in cases of myeloma.
[1]. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 2017 Revised 4th editionLyonIARC [Google Scholar]
[2]. Kintzer JS, Rosenow EC, Kyle RA, Thoracic and pulmonary abnormalities in multiple myeloma: A review of 958 casesArch Intern Med 1978 138:727-30.10.1001/archinte.1978.03630290039015646535 [Google Scholar] [CrossRef] [PubMed]
[3]. Al-Farsi K, Al-Haddabi I, Al-Riyami N, Al-Sukaiti R, Al-Kindi S, Myelomatous pleural effusion. Case report and review of the literatureSultan Qaboos Univ Med J 2011 11(2):259-64. [Google Scholar]
[4]. Inoue Y, Chua K, McClure RF, Jimenez MC, Gocke CD, Badros AZ, Multiple myeloma presenting initially as a solitary pleural effusion later complicated by malignant plasmacytic ascitesLeuk Res 2005 29:715-18.10.1016/j.leukres.2004.12.00515863214 [Google Scholar] [CrossRef] [PubMed]
[5]. Dhingra KK, Singhal N, Nigam S, Jain S, Unsuspected multiple myeloma presenting as bilateral pleural effusion- A cytological diagnosisCytojournal 2007 4:17-19.10.1186/1742-6413-4-1717825100 [Google Scholar] [CrossRef] [PubMed]
[6]. Yokoyama T, Tanaka A, Kato S, Aizawa H, Multiple myeloma presenting initially with pleural effusion and a unique paraspinal tumor in the thoraxIntern Med 2008 47:1917-20.10.2169/internalmedicine.47.129618981637 [Google Scholar] [CrossRef] [PubMed]
[7]. Oudart JB, Maquart FX, Semouma O, Lauer M, Demoulin PA, Ramont L, Pleural effusion in a patient with multiple myelomaClin Chem 2012 58:672-74.10.1373/clinchem.2010.16099422461512 [Google Scholar] [CrossRef] [PubMed]
[8]. Psallidas I, Kalomenidis I, Porcel JM, Robinson BW, Stathopoulos GT, Malignant pleural effusion: From bench to bedsideEur Respir Rev 2016 25:189-98.10.1183/16000617.0019-201627246596 [Google Scholar] [CrossRef] [PubMed]
[9]. Leach M, Drummond M, Doig A, Practical flow cytometry in haematology diagnosis 2013 UKJohn Wiley & Sons, Ltd10.1002/9781118487969 [Google Scholar] [CrossRef]
[10]. Babu GK, Saldanha SC, Lokesh KN, Suresh Babu MC, Patil AD, Kiran PR, Myelomatous pleural effusion: A rare case entity reported from a tertiary care cancer center in South IndiaLung India 2017 34(2):176-78.10.4103/0970-2113.20131828360469 [Google Scholar] [CrossRef] [PubMed]
[11]. Rodriguez JN, Pereira A, Martinez JC, Conde J, Pujol E, Pleural effusion in multiple myelomaChest 1994 105:622-24.10.1378/chest.105.2.6228306782 [Google Scholar] [CrossRef] [PubMed]
[12]. Waddell CC, Response of myelomatous pleural effusion to chemotherapyChest 1981 80:06-07.10.1378/chest.80.6.765b7307607 [Google Scholar] [CrossRef] [PubMed]
[13]. Kim YJ, Kim SJ, Min K, Kim HY, Kim HJ, Lee YK, Multiple myeloma with myelomatous pleural effusion: A case report and review of the literatureActa Haematol 2008 120:108-11.10.1159/00016569418957845 [Google Scholar] [CrossRef] [PubMed]
[14]. Ghosh S, Gopal R, Advani SH, Myelomatous pleural effusionJ Assoc Physicians India 2006 54:738-39. [Google Scholar]
[15]. Munshi NC, Anderson KC, DeVita Hellman Rosenberg Cancer: Principles and Practice of Oncology 2015 10th edPhiladelphiaLippincott Williams and Wilkins [Google Scholar]
[16]. Abhishek K, Ejazi MA, Hashim Z, Chaudhary R, Niharika K, Multiple myeloma with different thoracic manifestations: Case seriesIndian J Respir Care 2018 7:108-12.10.4103/ijrc.ijrc_11_18 [Google Scholar] [CrossRef]