The COVID-19 is an acute viral infection caused by a new coronavirus named as SARS-CoV-2. It was first identified in December 2019 in Wuhan, China. The World Health Organisation (WHO) declared the outbreak a Public Health Emergency of International Concern in January 2020 and a pandemic on March 10th 2020 [1]. It presents with diverse clinical symptoms like sore throat, cough, fever, myalgia, loss of smell and taste and breathlessness and higher risk of death in hospitalised patients [2].
Transmission of COVID-19 mainly occurs through droplets or aerosol as an infected person breathes, coughs, sneezes, sings, or speaks. Transmission of infection is possible when there is contact with contaminated surfaces [3]. It can spread as early as two days before infected persons show symptoms, and also from asymptomatic persons. People remain infectious for up to 10 days in moderate cases, and two weeks in severe cases. Pneumonia and Acute Respiratory Distress Syndrome (ARDS) are the complications which result in increased morbidities and mortalities. The incubation period has been found to be as long as 19 or 24 days although the case definitions typically rely on a 14-day window period [4].
The standard test for diagnosis of COVID-19 is to detect the presence of SARS-CoV-2 by RT-PCR testing of respiratory secretions collected using a nasopharyngeal and oropharyngeal swab which detects the presence of viral RNA fragments [5]. Rapid Antigen Test (RAT) to detect antigen and ELISA, Chemiluminescence Assay (CLIA) and Immunochromatography to detect the antibodies are the other tests available. Antigen detection assays have been developed to detect viral proteins in biological specimens such as nasopharyngeal secretions from infected patients [6]. Many ELISA-based assays have been developed to evaluate the human antibody response (IgA, IgM, and IgG) against SARS-CoV-2 [7]. Virus culture and isolation and gene sequencing are being carried out at research laboratories. Human airway epithelial cell lines were used for the initial isolation of the virus [8]. Whole genome sequencing was used to identify potential aetiological agents involved in the index cases of the COVID-19 pandemic in Wuhan [9].
Serologic assays like IgM, IgA and IgG antibodies for SARS-CoV-2, play an important role in understanding the epidemiology of COVID-19 in HCWs and in the general population. This also helps in identifying vulnerable groups at higher risk for infection. The implication of antibody test is to determine whether the individual being tested has recent or past infection even if that person is asymptomatic. The researchers have found three types of antibodies in the blood samples of patients infected with COVID-19. The first type of immunoglobulin isotype which induces sustained immunity is IgG. The secretory IgA synthesised by mucosa confers mucosal immunity and are found in high levels in tears, mucus, and other body fluids. The third type is Immunoglobulin M (IgM), which is the first antibody produced in any kind of infection. Because IgA and IgM antibodies disappear earlier than IgG, testing for these different antibody types help to distinguish between recent and past infection. Certainly, this can impart knowledge on the antibody response in infected individuals, spread of infection in the community and about herd immunity. A number of immunoassays based on ELISA and Immunochromatography have been designed to detect COVID-19 antibodies and antigen. Detection of a high titre of IgG antibody by ELISA indicates the presence of neutralising antibodies [10].
Globally, COVID-19 pandemic poses a high risk for HCW who are among the population that is most vulnerable of being infected with COVID-19. Health care workers are at exceptionally high risk of infection as they are the front liners of the current pandemic [11]. With a prevailing pandemic such as COVID-19, it becomes important to understand the presence and persistence of antibodies in the serum of HCW testing positive for COVID-19 on RT-PCR. It has been concluded from a study conducted on thousands of HCWs in England that chances of getting re-infection is very less at least for few months in those HCWs who were COVID-19 IgG antibodies positive. SARS-CoV-2 specific IgG levels remained constant for nearly 82 days following symptom onset in some patients [12]. The information related to COVID-19 acquired immunity found on a pre-print on medRxiv, shows that the individuals with COVID-19 IgG antibodies are protected from re-infection for six months or more than six months [13].
An understanding of the prevalence of IgG antibodies against COVID-19 and the duration for which they are present in the serum of HCWs will help in predicting the immune response of individuals against the disease and whether these antibodies are protective enough in preventing re-infection. Regular follow-up of these HCWs will reveal how long these antibody positive HCWs are protected from re-infection. Hence, this study was designed to find out the seroprevalence of COVID-19 IgG antibodies in RT-PCR positive symptomatic, asymptomatic and RT-PCR negative HCWs and the average period the antibodies persisted.
Materials and Methods
A longitudinal study was conducted for a period of nine months from April 2020 to December 2020. Sample size was 90 based on a pilot study. The study was approved by Institutional Ethics Committee, Government Kilpauk Medical College, Chennai, Tamil Nadu, India (vide Protocol ID.No.409/2020 Reg.No.ECR/1385/Inst/TN/2020). Informed written consent was obtained from the HCWs before recruiting them for the study. The study enrolled 45 HCWs with COVID-19 infection with or without symptoms confirmed by RT-PCR of nasopharyngeal and oropharyngeal swabs and 45 HCWs who were RT-PCR negative. Among the RT-PCR positive subjects 33 were symptomatic and 12 were asymptomatic. A minimum period of 15 days after being tested positive in RT-PCR was the criteria to recruit the subjects for the study.
Inclusion criteria: Laboratory confirmed COVID-19 RT-PCR positive subjects with any one of the following symptoms like fever, sore throat, cough, myalgia, anosmia, breathlessness and laboratory confirmed COVID-19 RT-PCR positive subjects with Household (H/O) contact with COVID-19 RT-PCR positive subjects. Age and sex matched RT-PCR negative healthy controls were also included.
Exclusion criteria: Subjects with allergic rhinitis and asthmatic bronchitis were excluded from the study population.
Study Procedure
Under strict aseptic precautions, 3 mL of blood was collected in plain tubes without anticoagulants, centrifuged at 1500 rpm for five minutes and serum was separated. Sera were stored at -20°C deep freezer. The ELISA kit used to detect COVID-19 IgG antibody was COVID Kawach IgG Microlisa technology transfer from National Institute of Virology, Pune procured from J. Mitra & Co. Pvt., Ltd., New Delhi, India. On the day of the test, the reagents and samples were brought to room temperature. An inhouse positive control serum from a COVID-19 patient that had high IgG titres was included for quality control to assess the performance of the kit. The principle of the test as per the kit insert is IgG antibodies present in serum bind to the SARS-CoV-2 virus whole cell antigen coated on to the wells on Micro titre plate. The enzyme linked conjugate binds to IgG antibodies captured. In the next step, Tetra Methyl Benzidine (TMB) is added and finally stop solution is added. By using an ELISA reader which was set at 450 nm, the optical density was measured for controls and samples. Based on the optical density, the antibody index was calculated for all the samples.
Interpretation
According to the kit protocol, the test was considered valid if the P/N ratio of Positive control was greater than 1.5:
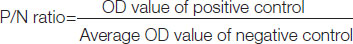
Antibody index of ≥1 Positive
Antibody index of <1 Negative
Statistical Analysis
Chi-square test was done to find out the statistical significance of COVID-19 IgG antibodies among RT-PCR positive and negative subjects. Likewise, Chi-square test was deployed to assess the statistical significance of COVID-19 IgG antibodies in COVID-19 RT-PCR positive symptomatic and asymptomatic subjects. Mean antibody index of symptomatic and asymptomatic subjects was calculated. Average number of days the antibodies persisted from the day of positive RT-PCR test to positive antibody test was calculated. Pearson correlation was run to find out the correlation between Ct value and antibody index.
Results
A total of 90 HCWs of age between 18-58 years were recruited for the study. Among the 45 COVID-19 RT-PCR positive HCWs, 16 (35.55%) were male and 29 (64.44%) were female (same in control group). The age and gender distribution of COVID-19 RT-PCR positive HCWs is given in [Table/Fig-1]. HCWs between 39 and 58 years were predominantly affected by COVID-19 compared to young adults.
Age and gender distribution of COVID-19 RT-PCR positive Healthcare workers (HCWs).
| Age (years) | Male n (%) | Female n (%) |
|---|
| 18-28 | 2 (12.50) | 3 (10.34) |
| 29-38 | 4 (25) | 7 (24.14) |
| 39-48 | 5 (31.25) | 9 (31.04) |
| 49-58 | 5 (31.25) | 10 (34.48) |
| Total | 16 (100) | 29 (100) |
Total n=45
Reverse transcription polymerase chain reaction was positive in 12 HCWs who were asymptomatic, but they had definite history of contact with laboratory confirmed cases. The clinical features observed in COVID-19 RT-PCR positive subjects are depicted in [Table/Fig-2]. Among the symptomatic group, fever with sore throat was the most common symptom observed. Fever was present in all symptomatic individuals except for one who presented with sore throat alone. Diarrhoea was the least common presenting symptom in this study.
Clinical features of COVID-19 RT-PCR positive Healthcare Workers (HCWs) (n=45).
| Symptoms | Number (n) | Percentage (%) |
|---|
| Asymptomatic | 12 | 26.66 |
| Fever with sore throat | 11 | 24.44 |
| Fever with cough | 8 | 17.77 |
| Fever with myalgia | 7 | 15.55 |
| Fever alone | 5 | 11.11 |
| Fever with diarrhoea | 1 | 2.22 |
| Sore throat | 1 | 2.22 |
The presence of COVID-19 IgG antibody among COVID-19 RT-PCR positive HCWs and RT-PCR negative HCWs was compared and the results are summarised in [Table/Fig-3]. The average number of days that the antibodies persisted from the day the HCW became RT-PCR positive was 104 days and the range was 25-266 days. Mean IgG antibody index was 3.19 and the range was 1.158-8.229 in IgG positive subjects.
IgG positive among RT-PCR positives vs RT-PCR negatives.
| RT-PCR | IgG Positive | IgG Negative | Total |
|---|
| Positive | 37 | 8 | 45 |
| Negative | 12 | 33 | 45 |
| Total | 49 | 41 | 90 |
The Chi-square is 27.999; p-value is <0.0001*; Significant at p<0.05
Out of the eight, COVID-19 RT-PCR positive but COVID-19 IgG antibody negative subjects, 4 (50%) were asymptomatic and 4 (50%) were symptomatic. Antibody test was carried out after a minimum period of 15 days from the day of RT-PCR test.
Twelve subjects in the present study exhibited COVID-19 antibodies despite being RT-PCR negative. This may be due to suspected or confirmed contact with COVID-19 positive patients. Out of these, six of the laboratory technicians who were engaged with sample collection and RT-PCR testing for COVID-19 were RT-PCR negative, but antibody positive. Similarly, three Doctors involved in COVID-19 diagnostic activities and two Physicians posted in COVID-19 ward were COVID-19 antibody positive but RT-PCR negative. One data entry operator posted at Microbiology laboratory for COVID-19 data entry work was RT-PCR negative but antibody positive. History of definite contact with laboratory confirmed case was present in four of the six technicians who were antibody positive. Among the five doctors who tested positive for COVID-19 IgG antibody two of them had mild fever for three days and one had contact history. The relationship between Ct value of Ribonucleic Acid (RNA)-dependent RNA POLYMERASE (RdRp) and Mean antibody index was calculated and this is depicted in [Table/Fig-4].
Cycle Threshold (Ct) vs antibody index.
| Ct value (RdRp) | Number | Mean IgG antibody index |
|---|
| 10-15 | 11 | 4.012 |
| 15-20 | 16 | 3.540 |
| 20-25 | 7 | 3.023 |
| 25-30 | 3 | 2.401 |
Cycle threshold value was found to be inversely proportional to mean antibody index. Ct value indirectly denotes the viral load. Individuals with low Ct value has high viral load and because of their strong immune response the antibody index is high whereas subjects with high Ct values had low antibody index. Pearson Correlation Coefficient is -0.9979 and it denotes strong negative correlation.
A comparison was made to find out the prevalence of COVID-19 IgG antibody in symptomatic and asymptomatic COVID-19 RT-PCR positives and is given in [Table/Fig-5].
IgG positive among asymptomatic vs symptomatic RT-PCR positives.
| RT-PCR Positive | IgG Positive | IgG Negative | Total |
|---|
| Asymptomatic | 8 | 4 | 12 |
| Symptomatic | 29 | 4 | 33 |
| Total | 37 | 8 | 45 |
The Chi-square statistic is 2.7088; p-value is 0.9973; Not significant at p<0.05; Mean antibody index in symptomatic and asymptomatic is 3.7743±1.9834 and 3.571±1.7961, respectively
COVID-19 IgG antibodies among RT-PCR positive symptomatic, asymptomatic and RT-PCR negative subjects is depicted in [Table/Fig-6]. Higher percentage of COVID-19 IgG antibodies were found in symptomatic COVID-19 subjects compared to asymptomatic group.
Prevalence of IgG antibodies among the three groups.
| RT-PCR | Number | Percentage (%) |
|---|
| RT-PCR (+) symptomatic (n=33) | 29 | 87.87 |
| RT-PCR (+) asymptomatic (n=12) | 8 | 66.66 |
| RT-PCR (-) (n=45) | 12 | 26.66 |
Discussion
Healthcare workers are more vulnerable to COVID-19 than the general population as they are the frontline workers who are in frequent contact with infected individuals. According to WHO report, one in ten health workers is infected with SARS-COV-2 in certain countries [14]. In Italy among those affected by COVID-19 infection, 9% were contributed by HCWs as of March 2020 [15].
Fever with sore throat was the chief complaint in 11 (24%) of the HCWs in the present study. About 8 (17%) of the HCWs were presented with fever and cough. Diarrhoea was the least common symptom observed. In our healthcare setting, around one in four HCWS who tested COVID-19 RT-PCR positive were asymptomatic. The asymptomatic cases were of age group between 18-30 years. HCWs above 30 years presented with either of the symptoms discussed above. The symptoms of COVID-19 are variable, but often include fever, sore throat, cough, fatigue, loss of smell and taste and breathing difficulty. Around one in five infected individuals do not develop any symptoms [16]. The index study is in accordance with this in terms of clinical presentation.
In this study, 82% of the COVID-19 RT-PCR positive HCWs were COVID-19 IgG antibody positive. It has been demonstrated that after two weeks post-disease onset, the average seroconversion rate is between 90-100% for IgM-IgG antibodies produced against the SARS-CoV-2 S and N proteins [17,18]. A study from a Tertiary Academic Hospital in New York City showed 36% of HCW had IgG antibodies to SARS-CoV-2, reflecting the high exposure of inpatient and ambulatory frontline staff to this viral illness, most of whom had minimal symptoms and were working in the weeks preceding testing [19].
In an outbreak at the University perinatal centre in Regensburg, Germany, a total of 36 staff tested positive for COVID-19 by RT-PCR and 34 of them developed symptoms [20]. The initial antibody testing by the University showed that 2-3 weeks after the initial PCR based screening only a limited number of staff members affected by COVID-19 had developed relevant antibody responses (48.4%). Premkumar, L et al., did a comparative study in patients and healthy controls to find out the immune responses to receptor binding domain of spike proteins of SARS-CoV-2 by ELISA test [21]. The study proved that 98% of the subjects developed IgG antibodies nine days after symptom onset and the specificity was 100%.
In this study, for all the HCWs who tested positive in RT-PCR, blood sample for IgG antibody test was taken after a minimum period of 15 days from the day of RT-PCR test. Probably this might be the reason for the high prevalence of COVID-19 IgG antibodies in HCWs. Racial difference, environmental factors and genetic makeup of the individuals can be the factors which modify the immune response in diverse population. Centre for Disease Control (CDC) and COVID-19-Associated Hospitalisation Surveillance Network reported that among the COVID-19 positive subjects included in the study, 45%, 33%, 8%, 5%, and <1% were Caucasian, African-American, Hispanic, Asian and American Indian/Alaskan native, respectively. Rest of the 7.9% were contributed by other or unknown race [22].
Among the RT-PCR positive HCWs, 8 (17.77%) tested negative for COVID-19 IgG antibody in this study. This negative IgG antibody response may be probably due to antibody decay, waning of antibody response and short-term immune response. One HCW who was just 21 days from symptom onset and RT-PCR positive did not develop IgG antibody in the present study. This might be due to failure of antibody production rather than antibody decay. Some of them were negative for IgG antibody 108 days after positive RT-PCR test which could be due to waning of antibody response. It was observed during an outbreak that few COVID-19 patients failed to produce any kind of antibodies one to two months following symptom onset [23-25].
On analysing the Ct value of RT-PCR positive samples, a negative correlation was found between Ct value and antibody index in the present study. Lower the Ct value, higher the antibody indexes is an indirect evidence of high viral load. Zou L et al., monitored SARS-CoV-2 viral load of nasal and oropharyngeal swabs from 18 patients in relation to the symptom onset [26]. Lower Ct and higher viral load were detected soon after the symptoms onset. Bullard J et al., examined the relationship between SARS-CoV-2 RT-PCR Ct values from respiratory samples, Symptom onset to Test (STT) and infectivity in vero cell lines culture [27]. Viral growth was seen in only 28.9% of samples. Interestingly, there was no growth in samples with a Ct >24 or STT >8 days. They concluded that infectivity may be low in patients with Ct >24 and duration of symptoms longer than eight days [28].
In the early period of COVID-19 pandemic, it was believed that lasting immunity against COVID-19 was present only in people who presented with severe infection. But now, a new study claims that even asymptomatic people can have long-lasting immunity from the coronavirus. A study on the evaluation of antibody responses in asymptomatic COVID-19 subjects at varied period of times proved that 71% and 57.1% were ELISA positive at two months and five months, respectively after infection, and they also had neutralising antibodies [29]. In a study conducted at a tertiary care centre in Delhi follow-up of a few of the seropositive participants revealed that antibodies against the infection lasted for 60-80 days, which is the maximum duration of follow-up that could be done [30]. A similar study from a tertiary care centre in Mumbai has shown that IgG antibodies persisted in COVID-19 positive HCWs for more than six months [Table/Fig-7] [30-33].
Previous literature on antibody persistence in COVID-19 population [30-33].
| Author Name, [Reference] | Place | Study population | Days of antibody persistence |
|---|
| Siddiqui S et al., [30] | New Delhi | 780 | 83 days |
| Singhal T et al., [31] | Mumbai | 244 | More than 6 months |
| Iyer AS et al., [32] | Boston | 343 | ≤122 days |
| Isho B et al., [33] | Toronto, Canada | 439 | More than 3 months |
| Present study | Chennai | 90 | 266 days |
The study conducted on contacts with diseased staff showed they were RT-PCR negative but IgA was positive in 8.2% and one had IgG antibody which was slightly elevated above the normal limit [34]. In the present study, 66.66% of the asymptomatic RT-PCR positive were found to be COVID-19 IgG antibody positive. There was no statistical significance of COVID-19 IgG antibody between symptomatic and asymptomatic group. The mean antibody index in asymptomatic was 3.571±1.796 which is slightly lower than the symptomatic individuals. Long QX et al., did a study to compare the immune responses between asymptomatic and symptomatic RT-PCR positive COVID-19 subjects. The study revealed 84% of asymptomatic were IgG positive 15 days to one month after exposure. However, in the above study the IgG titre was lesser than in the symptomatic group [34]. Therefore, the inference on antibody production by asymptomatic laboratory confirmed RT-PCR positive group in the present study is in concordance with the above study.
A laboratory technician contracted the disease from her son who returned from United Kingdom in February 2020. Four of the family members who were in close contact with the index case became symptomatic and RT-PCR positive. All of them were hospitalised and discharged after 21 days when the repeat RT-PCR test was found negative. The antibody test revealed a high antibody index in all of them even after 266 days of symptom onset. None of them got re-infection till date. From this finding it can be ascertained that the IgG antibody present confers immunity against re-infection with SARS-CoV-2 for more than eight months in some of the cases. However, two of the laboratory technicians who were RT-PCR and COVID-19 IgG antibody positive suffered from parotitis two months after symptom onset. Those two were negative in repeat RT-PCR test for SARS-CoV-2. It could be a post COVID complication Many otolaryngologists have observed an increase in the number of patients with acute parotitis (inflammation of the parotid salivary glands), which could be related to COVID-19 [35]. Few HCWs developed fever, excruciating low backache and breathlessness in the subsequent months but they were all negative in repeat test. Involvement of musculoskeletal system, joints and nervous system has been found in patients with moderate and severe disease [36]. Inflammation in COVID-19 is not restricted to respiratory tract alone. Systemic inflammation can occur in any organ or system [37].
From this study, it is deduced that the IgG antibody persists for few months in COVID-19 patients and has a definite role in preventing re-infection for few months at least.
Limitation(s)
As the resources are limited, sample size was small and therefore it is difficult to draw a conclusion on the role of IgG in preventing re-infection with SARS-CoV-2. The study does not include testing for the cross-reacting antigens of SARS-CoV-1, Middle east respiratory syndrome coronavirus or other human coronaviruses that circulate and infect humans. The IgG ELISA test was carried out at one point of time only. In RT-PCR positive, COVID-19 IgG negative HCWs, a repeat antibody test was not done and so false negative could not be ruled out. Similarly, in the RT-PCR negative, antibody positive subjects’ false positive result cannot be ruled out. Despite the above limitations, the study can be applied as a tool in epidemiological studies and to determine IgG antibody response to SARS-CoV-2 in HCWs.
Conclusion(s)
From the data derived from this study, it can be concluded that the prevalence of COVID-19 IgG antibodies in RT-PCR positive symptomatic was high compared to asymptomatic RT-PCR positive subjects. Average number of days that the antibody persisted was 104 days post PCR test and the maximum number of days antibodies persisted was 266 days. Therefore, we recommend all the HCWs to follow the standard precautions of prevention of COVID-19 infection. Further studies and follow-up of these HCWS are necessary to determine how long the protective response persists and prevent re-infection in those infected with COVID-19. This study has a definite role in seroepidemiological studies.
Total n=45
The Chi-square is 27.999; p-value is <0.0001*; Significant at p<0.05
The Chi-square statistic is 2.7088; p-value is 0.9973; Not significant at p<0.05; Mean antibody index in symptomatic and asymptomatic is 3.7743±1.9834 and 3.571±1.7961, respectively