T2 Weighted Hypointense Subcortical White Matter- A Rare Imaging Feature in Ketotic Hyperglycaemia
Rudresh Hiremath1, Vinyasa Nagesh2, Divya Vishwanatha Kini3
1 Professor, Department of Radiology, Jagadguru Sri Shivarathreeshwara Medical College, Mysore, Karnataka, India.
2 Junior Resident, Department of Radiology, Jagadguru Sri Shivarathreeshwara Medical College, Mysore, Karnataka, India.
3 Junior Resident, Department of Radiology, Jagadguru Sri Shivarathreeshwara Medical College, Mysore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Divya Vishwanatha Kini, 119, Mahamaya Krupa, 12th Cross, 4th Main, Jayanagar, Mysore, Karnataka, India.
E-mail: dillakini@gmail.com
Diabetic ketoacidosis is a hyperglycaemic state in which serum glucose level is more than 250 mg/dL, a pH less than 7.3, a serum bicarbonate level less than 18 mEq/L, and serum ketone level is raised and dehydration is present. Insulin deficiency is the main precipitating factor. Rarely, they present with neurological complications such as focal/generalised motor seizures, hemiparesis and sensory deficits which may be associated with various imaging features. With increasing prevalence of diabetes in the last decade, it is vital to familiarise with various hyperglycaemia induced imaging abnormalities in brain in order to provide accurate diagnosis for providing prompt treatment and preventing complications. T2 Weighted (T2W)/ Fluid-Attenuated Inversion Recovery (FLAIR) subcortical hypointensity in ketotic patients presenting with focal seizures had been reported rarely. Here, authors presented the imaging findings of a 27-year-old female patient with newly diagnosed type II diabetes mellitus and diabetic ketoacidosis with new onset seizures. T2 subcortical white matter hypointensity was noted in the right post-central gyrus. Though non specific, this imaging finding was of importance in such cases of hyperglycaemia in order to formulate further patient care.
Diabetes, Magnetic resonance imaging, Seizures
Case Report
A 27-year-old female patient, known case of hyperthyroidism since five years and treatment defaulter for the past 15 days (Carbimazole), presented with complaints of weight loss (~12 kg) over the past 1.5 months, generalised weakness for 15 days and 3-4 episodes of vomiting since three days. No prior history of other co-morbidities was noted. On presentation, she was found to be dehydrated and appeared cachexic. However, systemic examination was found to be normal. Initial work-up showed an extremely elevated random blood glucose levels >500 mg/dL with large quantities of urine ketone bodies (3+). Glycosylated haemoglobin of 12.5 g% with elevated total counts of 14,000 was observed. Mild hyponatraemia (134 mEq/L) and hypokalaemia (3.5 mEq/L) was noted.
A clinical diagnosis of diabetic ketoacidosis was made and patient was shifted to the Intensive Care Unit (ICU) and started on insulin infusion (6 units/hr) with a cover of broad-spectrum antibiotics, electrolyte imbalance corrected and monitored at regular intervals. Portable chest radiograph was performed and was normal. Patient improved symptomatically over the next 3 days and was further shifted to the ward. Here, 3 days post ICU stay, she developed new onset focal seizures in the form of twitching of the left side of the face; however, no Electroencephalogram (EEG) was performed. No other abnormal limb movements or neurological deficits were noted. Patient was immediately started on antiepileptics (levitiracetam 10 mg/kg Intravenous (i.v) and phenytoin 15 mg/kg IV) but further developed another episode for which medications were up-scaled (Inj Levitiracetam 750 mg 1-0-1 and Inj Phenytoin 100 mg 1-1-1). Capillary blood glucose levels during both episodes were elevated to ~300-400 mg/dL.
MRI brain (by 3 Tesla machine) was done to rule out organicity, which revealed focal subcortical white matter hypointensity on Time (T) T2 [Table/Fig-1,2a,b] FLAIR sequences in the right post-central gyrus with subtle blooming on Gradient Echo (GRE) sequences [Table/Fig-2c]. There was mild diffusion restriction confirmed by Apparent Diffusion Coefficient (ADC) maps. Lesions were isointense on T1 weighted images [Table/Fig-3a,b,4]. No other focal abnormalities or similar lesions were noted in the study [Table/Fig-1,2,3 and 4]. Possibility of sub-acute infarct, infectious and other vascular aetiologies were considered. Correlation with the typical imaging features and further lack of clinical correlation, the possibilities were ruled out.
Axial T1 weighted image appears normal with normal grey-white matter differentiation. T: Time
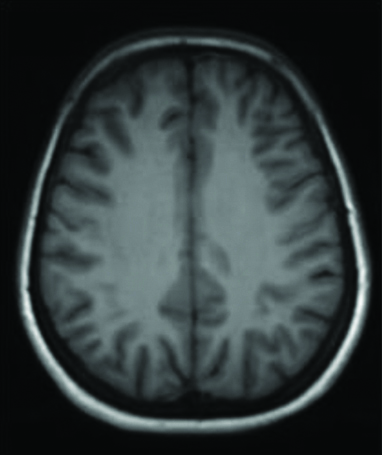
Axial T2 weighted (a and b) and FLAIR (c) images demonstrate focal subcortical white matter hypointensity in the right post-central gyrus FLAIR: Fluid-attenuated inversion recovery

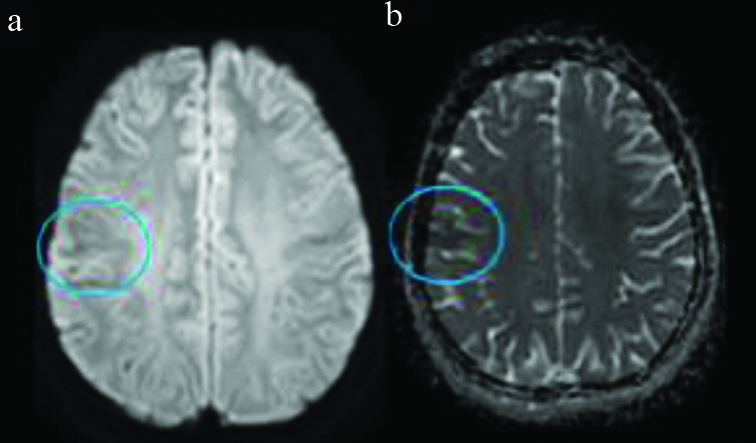
Axial DWI-ADC images. Evidence of mild diffusion restriction (a) with corresponding low ADC values (b) noted in the regions of previously demonstrated subcortical T2 hypointensity in the right postcentral gyrus.
DWI-ADC: Diffusion weighted images-apparent diffusion coefficient
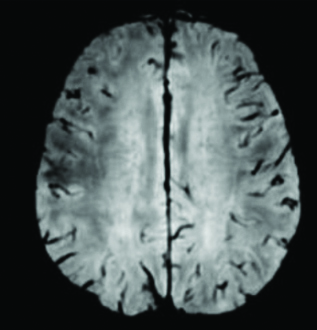
A 3T MRI - Axial gradient echo sequence images with evidence of subtle blooming in the regions of previously described subcortical T2 hypointensity in the right precentral gyrus.
MRI: Magnetic resonance imaging
Patient however left against medical advice with antiepileptics (Inj Levitiracetam 750 mg 1-0-1, Inj Phenytoin 100 mg 1-1-1 and Inj lacosamide 100 mg 1-0-1), insulin (Inj H. actrapid Subcutaneous 20-20-20) and Neomercazole (10 mg 1-0-0) for the hyperthyroidism continued as per advice on discharge. Patient was then re-admitted two days post-discharge with new complaints of decreased generalised and verbal responsiveness, new onset symptoms of involuntary movements of tongue with uprolling of eyes followed by post-ictal confusion. On examination, she was found to be drowsy but arousable with bilateral sluggishly reactive pupils. Same line of management was re-started, patient improved symptomatically and haemodynamically and was further discharged. On discharge, medications were continued for the next month (Tab Levitiracetam 750 mg 1-0-1, Tab Neomarcazole 10 mg 1-0-1, Inj H.Actrapid S/C 25-25-25-30 min b/f, Inj Lantus S/C 0-0-25-30 min after food). No new symptoms or complaints were noted in the follow-up visit after one month. Patient was seizure-free with good glycaemic control. Repeat MRI was not performed as patient was symptomatically stable with no new symptoms.
Discussion
Seizures are known to occur in metabolic disorders, but are considered rare in ketotic hyperglycaemia even when hyperglycaemia and hyperosmolality reach values comparable to those seen in non ketotic coma [1,2]. Surprisingly, in this case patient developed seizures.
Various pathophysiological postulates have been put forth as causes for hyperglycaemia induced seizure especially in patients with non ketotic hyperglycaemia. Maccario M concluded that rapid onset of hyperglycaemia and serum hyper-osmolality, leading to intracellular dehydration and disturbance of electrolyte equilibrium of the intracellular and extracellular compartments is the probable aetiologic factor [3]. Ozer F et al., concluded that hyperglycaemia increases Gamma-Aminobutyric Acid (GABA) metabolism and thereby diminish the seizure threshold [4]. Another hypothesis involves transient focal cerebral ischaemia caused by hyperglycaemia secondary to an increase in cerebrovascular resistance or loss of flow regulation [5].
In this case, there was hypointensity noted on T2 weighted images in the sub-cortical region in the pre-central gyrus with subtle blooming artifact and restricted diffusion which has been postulated as secondary to transitory accumulation of free radicals with iron. This is attributed to the axonal damage by excitotoxic activity during episodes of seizures in states of hyperglycaemia [6-9]. Hypointensity noted on T2 weighted images in the region has also been identified as a feature of early cortical ischaemia in animal studies [10].
Differentials have to be considered when considering such imaging features, in specific the subcortical T2 hypointensity. When considering vascular aetiologies, early sub-acute infarct and acute cerebral venous thrombosis are important differentials as both show evidence of T2 hypointensity. The presence of adjacent FLAIR hyperintense thrombosed cortical vein, significant diffusion restriction and absent flow related signal intensity on post-contrast images within the thrombosed vein helps rule out acute cerebral venous thrombosis. Early infarct was ruled out owing to the absence of significant restricted diffusion and well-delineated grey-white matter differentiation. Most importantly, clinical correlation is vital to the decision regarding the diagnosis. Certain other entities such as cerebral metastasis may mimic this pathology, however most neoplasms tend to appear hyperintense on T2 weighted images owing to their cellularity and strongly enhance on post-contrast images. The imaging features of hyperglycaemia induced seizure can be varied ranging from a normal MRI study to hypointensity on T2 weighted images in the sub-cortical region, cortical hyper intensity with restricted diffusion, and cortical or lepto-meningea post-contrast enhancement, predominantly involving parieto-occipital region [7,9].
Further studies have also shown a strong association of hyperglycaemia with focal as well as partial seizures with imaging demonstrating sub-cortical hypointensity in the occipital lobes. Patients in such states present with vision loss as the primary complaint [11]. Additionally, hyperperfusion was also demonstrated in these areas [12].
Other cases reported by Tsai JP et al., have depicted imaging findings similar to the case being presented here i.e., T2 and Susceptibility Weighted Imaging (SWI) hypointensity in an elderly patient presenting with focal motor seizures in a setting of non-keratotic hyperglycaemia, Additionally, with corresponding ribbon focal cortical enhancement was also noted [13]. Similar study was performed by Hiremath SB et al., wherein 83.3% of the patients presenting with hyperglycaemia induced seizures demonstrated evidence of subcortical T2 hypointensity [9]. Controlling blood sugar levels, correcting the dehydration and the dyselectrolytemia in addition to initiating insulin therapy plays a vital role as this had been shown to effectively control seizures. Seizures may even be aggravated by antiepileptic treatment [14,15].
Conclusion(s)
Neuroimaging features of hyperglycaemia induced seizures are varied. There are few causes of T2 hypointense lesions in cerebral parenchyma, and this condition adds to the list. Though non specific, the awareness of this kind of white matter hypointensities on MRI in hyperglycaemia induced seizures helps to facilitate early diagnosis, especially in young patients and prevent unnecessary work-up thereby help administer appropriate timely management.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jan 12, 2021
Manual Googling: Apr 19, 2021
iThenticate Software: May 05, 2021 (4%)
[1]. Trachtenbarg DE, Diabetic ketoacidosisAmerican Family Physician 2005 71(9):1705-14. [Google Scholar]
[2]. Guisado R, Arieff A, Neurologic manifestations of diabetic comas: Correlation with biochemical alterations in the brainMetabolism 1975 24(5):665-79.10.1016/0026-0495(75)90146-8 [Google Scholar] [CrossRef]
[3]. Maccario M, Neurological dysfunction associated with nonketotic hyperglycaemiaArchives of Neurology 1968 19(5):525-34.10.1001/archneur.1968.004800500950095684300 [Google Scholar] [CrossRef] [PubMed]
[4]. Ozer F, Mutlu A, Ozkayran T, Reflex epilepsy and non-ketotic hyperglycaemiaEpileptic Disorders 2003 5(3):165-68. [Google Scholar]
[5]. Duckrow RB, Beard DC, Brennan RW, Regional cerebral blood flow decreases during hyperglycaemiaAnnals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 1985 17(3):267-72.10.1002/ana.4101703083922283 [Google Scholar] [CrossRef] [PubMed]
[6]. Seo DW, Na DG, Na DL, Moon SY, Hong SB, Subcortical hypointensity in partial status epilepticus associated with nonketotic hyperglycaemiaJournal of Neuroimaging 2003 13(3):259-63.10.1111/j.1552-6569.2003.tb00188.x12889174 [Google Scholar] [CrossRef] [PubMed]
[7]. Lee EJ, Kim KK, Lee EK, Lee JE, Characteristic MRI findings in hyperglycaemia-induced seizures: Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery imagingClinical Radiology 2016 71(12):1240-47.10.1016/j.crad.2016.05.00627289324 [Google Scholar] [CrossRef] [PubMed]
[8]. Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, Transient MR signal changes in patients with generalised tonicoclonic seizure or status epilepticus: Periictal diffusion-weighted imagingAmerican Journal of Neuroradiology 2001 22(6):1149-60. [Google Scholar]
[9]. Hiremath SB, Gautam AA, George PJ, Thomas A, Thomas R, Benjamin G, Hyperglycaemia-induced seizures-Understanding the clinico-radiological associationThe Indian Journal of Radiology & Imaging 2019 29(4):343-49.10.4103/ijri.IJRI_344_1931949334 [Google Scholar] [CrossRef] [PubMed]
[10]. Ida M, Mizunuma K, Hata Y, Tada S, Subcortical low intensity in early cortical ischemiaAmerican Journal of Neuroradiology 1994 15(7):1387-93. [Google Scholar]
[11]. Putta SL, Weisholtz D, Milligan TA, Occipital seizures and subcortical T2 hypointensity in the setting of hyperglycaemiaEpilepsy & Behavior Case Reports 2014 2:96-99.10.1016/j.ebcr.2014.01.00125667880 [Google Scholar] [CrossRef] [PubMed]
[12]. Sasaki F, Kawajiri S, Nakajima S, Yamaguchi A, Tomizawa Y, Noda K, Occipital lobe seizures and subcortical T2 and T2* hypointensity associated with nonketotic hyperglycaemia: A case reportJournal of Medical Case Reports 2016 10(1):01-04.10.1186/s13256-016-1010-827520801 [Google Scholar] [CrossRef] [PubMed]
[13]. Tsai JP, Sheu JJ, Hsieh KL, Unusual magnetic resonance imaging abnormality in nonketotic hyperglycaemia-related epilepsia partialis continuaAnn Indian Acad Neurol 2018 21(3):225-27.10.4103/aian.AIAN_386_1730258268 [Google Scholar] [CrossRef] [PubMed]
[14]. Wang X, Nonketotic hyperglycaemia-related epileptic seizuresChinese Neurosurgical Journal 2017 3(1):01-04.10.1186/s41016-017-0073-8 [Google Scholar] [CrossRef]
[15]. Rani KA, Ahmed MH, Dunphy L, Behnam Y, Complex partial seizure as a manifestation of non-ketotic hyperglycaemia: The needle recovered from haystack?Journal of Clinical Medicine Research 2016 8(6):478-79.10.14740/jocmr2552w27222677 [Google Scholar] [CrossRef] [PubMed]