Characterisation of Candida Colonisation in Neonates
Abhilasha Dalal1, GR Jagannatha Babu2, K Anuradha3
1 Postgraduate Student, Department of Microbiology, Mysore Medical College and Research Institute, Mysore, Karnataka, India.
2 Assistant Professor, Department of Microbiology, Mysore Medical College and Research Institute, Mysore, Karnataka, India.
3 Professor and Head, Department of Microbiology, Mysore Medical College and Research Institute, Mysore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Abhilasha Dalal, Akaanksha Residency, Block A-117, 2203/49A, Sayyaji Rao Road, New Bamboo Bazar, Mysore, Karnataka, India.
E-mail: drabhilashadalal@gmail.com
Introduction
The occurrence of invasive fungal infections has increased significantly worldwide, premature infants in Neonatal Intensive Care Unit (NICU) along with other risk factors are at particular risk of these invasive fungal infections which lead to fungal septicaemia in newborns. Candidaemia is the most common form of invasive candidiasis associated with an unacceptably high mortality rates. Candida colonisation in neonates is considered the first step towards developing neonatal sepsis.
Aim
To determine the prevalence of Candida colonisation and its characterisation among neonates admitted in NICU.
Materials and Methods
The present study was a prospective cross-sectional study with 150 neonates included in the study. Swabs were taken from four different sites of each neonate and inoculated on Chocolate agar and Sabouraud’s Dextrose Agar (SDA) and incubated at 37°C for seven days. Candida species isolated were confirmed by gram stain, germ tube test, growth on Chromogenic (CHROM) agar and cornmeal agar. Statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 11.0.
Results
A total of 32 (21.3%) neonates had Candida colonisation. Twenty two (68.7%) were low birth weight and 24 (75%) were born premature. Perineum was the most common (56%) site of colonisation. Among Candida isolates, Candida tropicalis (63%) was the commonest followed by Candida parapsilosis (25%) and Candida glabrata and Candida albicans (6%). The risk factors identified were low birth weight, premature birth, use of antibiotics.
Conclusion
Colonisation of preterm and low birth weight neonates by Candida species is a major risk factor and needs attention to avoid dissemination and life threatening infection.
Candidaemia, Low birth weight, Neonatal intensive care units, Non-albicans Candida, Preterm neonate
Introduction
In the past two decades, there has been an increased incidence and prevalence of invasive fungal infections especially in immunocompromised and those requiring prolonged hospitalisation [1]. Among the long list of opportunistic fungi causing serious, life threatening infections, Candida species remain single most important cause of opportunistic mycosis worldwide, there was a 400% increase in candidaemia since the 1980s [2]. Since 2013, the Leading International Fungal Education (LIFE) portal has facilitated the estimation of the burden of serious fungal infections country by country for over 5.7 billion people (>80% of the world’s population) among which the global estimate for invasive candidiasis was ~700,000 cases globally [3]. Also, Candida species have been identified as the third most common cause of late-onset sepsis in NICU patients [4].
In humans, Candida species colonises regions including skin, oropharynx, lower respiratory tract, gastrointestinal tract, and genitourinary system. Colonisation with Candida species occurs early in life of a neonate due to transmission from mother by vaginal delivery or by health care worker and hospital environment [5]. Candida colonisation is an important risk factor for systemic infection in neonates. Multiple risk factors have been identified for making a neonate susceptible to systemic candidiasis, these factors are low birth weight, gestation <25 weeks, prior Candida colonisation, prolonged treatment in intensive care units, invasive monitoring techniques, systemic antibiotics and parenteral nutrition [4,6]. It is important to understand factors affecting normal colonisation of the neonate, as well as those factors that predispose to fungal invasiveness. Candidiasis covers a wide range of diseases from more superficial and milder clinical manifestations such as oesophageal or oropharyngeal candidiasis to serious infections including Blood Stream Infections (BSIs) and disseminated candidiasis spreading to multiple organs, which is associated with high mortality rate. Early initiation of aggressive therapy, with careful monitoring, can lead to a successful outcome.
As many studies suggest [7-10], colonisation is the primary step for candidaemia. Such a type of study to look for Candida colonisation prevalence and characterisation in NICU had not been carried out before in our institute so this study was undertaken.
Materials and Methods
The present prospective cross-sectional study was conducted in NICU of a tertiary care teaching hospital at Mysore between August 2018-December 2018 after taking approval from Institutional Research and Ethics Committee (EC REG: ECR/134/Inst/KA/2013/RR-16).
Inclusion criteria: The subjects included for the study were neonates admitted in NICU for more than 48 hours.
Exclusion criteria: Neonates with an already established invasive fungal infection were excluded.
Sample size calculation: Sample size was calculated using the below formula:
n=Z2P(1-P)/d2Where n is the sample size, Z is the statistic corresponding to level of confidence, Z value for 95% confidence is 1.96. P is expected prevalence (taken as 32.5% from the previous study done by Nazir A and Masoodi T [7]), and d is precision, taken as 5%.
n=1.96×1.96×0.325(1-0.325)/0.05×0.05Sample size was calculated as 100 (rounded-off), 150 subjects were included.
The detailed history was noted down on the proforma after taking consent from the parent. The samples (swabs) were collected from 150 neonates from different sites like perineal area, ear canal, throat and umbilicus with the help of sterile cotton tipped swabs pre-moistened with sterile saline. Different swabs were used for collection from different sites. The swabs were rubbed on the selected site with gentle pressure.
All swabs were inoculated immediately on chocolate agar and SDA, incubated at 37°C for seven days. Culture tubes and plates were examined daily for their morphological and colonial characters. Growth observed on chocolate agar and SDA was creamy, smooth, pasty colonies after 24-48 hours or at the end of seven days [Table/Fig-1] which was confirmed morphologically, gram positive budding yeast cells by gram’s stain were seen [Table/Fig-2]. In addition, blood culture records were looked if any blood sample from the study subjects i.e., of the neonates were received and checked for Candida growth and correlated with Candida colonisation.
Growth on SDA- Candida spp grow as white creamy mucoid colonies.
Gram stain of Candida albicans showing typical oval budding yeast cells (100x oil immersion).
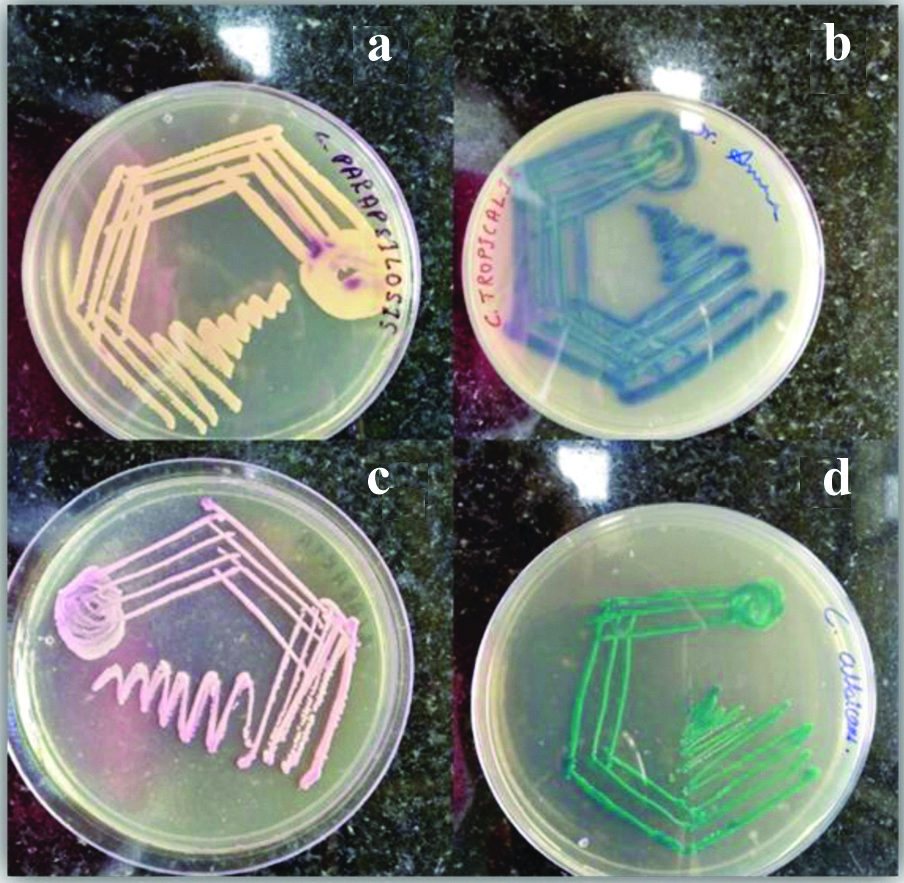
Identification of Candida species was done by its ability to form germ tube by Reynolds Braude phenomenon, hyphal, chlamydospore and blastospore formation by inoculating on to cornmeal agar and incubating for 24-48 hours and also growth on CHROM agar. Confirmed Candida colonies were inoculated on CHROM agar plates, incubated at 37°C and the plates were examined at 24 hours, 48 hours and 72 hours after incubation. After 24 hours of incubation majority of the Candida colonies had grown well but the accurate colour of the different species developed only after 48 hours of incubation [Table/Fig-3].
Colony morphology of Candida species on CHROMagar: (a) Candidaparapsilosis: cream to white smooth colonies; (b) Candida tropicalis: blue to metallic blue coloured raised colonies; (c) Candida glabrata: pink smooth colonies; (d) Candida albicans: light green coloured smooth colonies.
Statistical Analysis
Statistical analysis was done using SPSS version 11.0 and the prevalence of organisms was determined and expressed in percentage.
Results
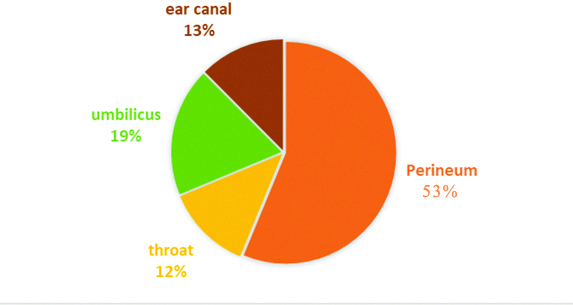
In the present study Candida colonisation was observed in 32 (21.3%) out of 150 neonates. Colonisation was found predominantly in perineal region in 17 (53%) out of 32 isolates, the colonisation in other sites is shown in [Table/Fig-4]. Among the various species isolated in colonised neonates Candida tropicalis was the common species followed by C.parapsilosisC.glabrata and C.albicans [Table/Fig-5].
Distribution of Candida colonisation in various sites.
Different Candida species isolated from the colonised subjects.
| Candida species | No. of isolates (%) |
|---|
| C.tropicalis | 20 (63) |
| C.parapilosis | 08 (25) |
| C.glabrata | 02 (06) |
| C.albicans | 02 (06) |
| Total | 32 (100) |
Age distribution among the colonised neonates, 2-10 days was predominant with 18 (56%) out of 32, total 26 (81%) of the affected neonates were below 30 days of age and 06 (19%) were above 30 days. The low birth weight 22 (55%) was most predominant risk factor, distribution of other risk factors in Candida colonised neonates are shown in [Table/Fig-6].
Risk factor identified in neonates with candidaemia.
| Variables | No. of subjects | Candida colonised n (%) |
|---|
| Place of delivery |
| Outborn | 60 | 19 (31.7) |
| Inborn | 90 | 13 (14.4) |
| Preterm | 87 | 24 (27.6) |
| Birth weight |
| LBW (2.5 kg-1.5 kg) | 40 | 22 (55) |
| ELBW (<1.5 kg) | 32 | 4 (12.5) |
| PROM | 56 | 14 (25) |
| Antibiotic therapy | 136 | 32 (23.5) |
LBW: Low birth weight; ELBW: Extremely low birth weight; PROM: Premature rupture of membranes
Blood culture data of neonates colonised with Candida was collected from the departmental blood culture register for correlation. It was found that Candida spp was isolated from blood culture among 14 out of the 32 Candida colonised neonates and same Candida spp was isolated from blood and swab in those neonates.
Discussion
Neonates represent a highly vulnerable patient population. It is estimated that invasive infections cause >1.4 million neonatal deaths worldwide annually [11]. There has been a significant increase in the incidence of invasive fungal infections worldwide especially in patients admitted in intensive care units. Among different fungal pathogens, colonisation by Candida species is very frequent in NICU and a necessary first step in the pathogenesis of systemic infection. Candida species are a common cause of neonatal nosocomial BSIs in premature infants and are a leading cause of infectious-related mortality in the NICU. The overall prevalence of Candida colonisation was 21.3% in neonates admitted to the NICU. Prevalence reported by other studies over the last decade point towards an increasing Candida prevalence, findings of these studies have been summarised in [Table/Fig-7] [7-10,12-18].
Candida prevalence reported by other studies [7-10,12-18].
| Study | Year | Place | Study population | Findings |
|---|
| Present study | 2021 | Karnataka, India | Neonates | Neonates positive for candidaemia-21% with C.tropicalis isolated as predominant species |
| Nazir A and Masoodi T [7] | 2018 | Kashmir, India | Neonates | Neonates positive for candidaemia-32.5% with C.tropicalis isolated as predominant species |
| Azim A et al., [8] | 2018 | Lucknow, India | Adults (ICU) | 95% unifocal and 81% multifocal Candida colonisation with C. glabrata 11 species isolated. |
| Latha GS et al., [9] | 2017 | Karnataka, India | Neonates | 13.8% neonates positive for Candida culture. Low birth weight identified as most common risk factor. |
| Pandita N et al., [10] | 2017 | Delhi, India | Neonates | Fungal sepsis in 13.6% neonates. C.glabrata most common species isolated, LBW and Prematurity most associated risk factors |
| Bansiwal K et al., [12] | 2016 | Rajasthan, India | Neonates | Candida colonisation rate was 47.31% in neonates with C.albicans most common isolated species |
| Abdallah Y et al., [13] | 2015 | Uganda | Neonates | Candida colonisation seen in 23.5% neonates. C.albicans most common isolated species, prematurity most common associated risk factor. |
| Mahnoudabadi AZ et al., [14] | 2015 | Iran | Neonates and Children | 34% oral swabs and 21% urine samples were positive for Candida species with C.albicans most common species isolated |
| Wadile RG and Bhate VM [15] | 2015 | Maharashtra, India | Neonates | Positive Candida culture in 32.26% neonates with C.albicans most common isolated species and LBW most common associated risk factor. |
| Noori Sanami M et al., [16] | 2015 | Iran | Neonates | 31.7% neonates positive for Candida culture. Rectum and umbilicus most common site for colonisation. |
| Tak V et al., [17] | 2014 | Delhi, India | Adults | 14.95% per 1000 ICU patients. C.tropicalis most common isolated species. |
| Sardana V et al., [18] | 2012 | Meerut, India | Neonates | 30.1% of neonates had candidemia with C.glabrata was the most common isolated species. |
In this study, most of the neonates had Candida colonisation by day ten and maximum colonisation was seen in perineum region. Several risk factors have been associated with Candida colonisation, major risk factors identified in this study were low birth weight, prematurity and use of antibiotic therapy. High percentage of the neonates with colonisation with Candida in present study had Low Birth Weight (LBW) (68%) and Extremely Low Birth Weight (ELBW) (13%) and 75% of them were born preterm, which is consistent with observations of other studies that the incidence of Candida colonisation was inversely proportional to birth weight and gestational age. This association of Candida colonisation and lower gestational age can be due to prematurity being associated with compromised immunity such as decreased skin and mucosal integrity, the relative quantitative deficiency of protective maternal IgA to Candida, also excessive handling of preterm neonates in neonatal units promotes horizontal acquisition [5]. Prolonged PROM (≥24hrs), especially in vaginally colonised mothers has been associated with neonatal septicaemia [19]. In present study, there was no association between Premature Rupture Of Membranes (PROM) and Candida colonisation was found, also the mothers with vaginal colonisation did not checked.
Historically, Candida albicans was the most frequently isolated species, but recently non-albicans Candida (NAC) species have emerged as important opportunistic pathogens [20]. In this study, the NAC species were the predominant organism responsible for colonisation in neonates and the remaining were of C. albicans. C.tropicalis was the most common species isolated.
Limitation(s)
The study had certain limitations. Blood samples did not collect from all the subjects to correlate the isolated coloniser with systemic infection. In addition, follow-up of candidemic neonates could not be done.
Conclusion(s)
In conclusion, in the present study it was observed that the neonates with risk factors have increased chance of being colonised with Candida in turn they may progress to candidaemia, which may increase the hospital stay, add socio-economic burden and increased morbidity and mortality.
Hence, focus should be shifted on prevention of candidaemia in these high-risk neonates by screening them for Candida colonisation, selectively keeping under observation and if suspected candidaemia they can start with antifungal agents instead of giving empirical treatment to all. Also, preventive measures like screening of NICU staff for carriers, restricted antibiotic use can decrease Candida colonisation and hence its infection.
LBW: Low birth weight; ELBW: Extremely low birth weight; PROM: Premature rupture of membranes
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jan 25, 2021
Manual Googling: Apr 22, 2021
iThenticate Software: May 25, 2021 (10%)
[1]. Pfaller MA, Diekema DJ, Epidemiology of invasive candidiasis: A persistent public health problemClin Microbiol Rev 2007 20(1):133-63.10.1128/CMR.00029-0617223626 [Google Scholar] [CrossRef] [PubMed]
[2]. Blot S, Dimopoulos G, Rello J, Vogelaers D, Is Candida really a threat in the ICU?Curr Opin Crit Care 2008 14:600-04.10.1097/MCC.0b013e32830f1dff18787456 [Google Scholar] [CrossRef] [PubMed]
[3]. Bongomin F, Gago S, Oladele RO, Denning DW, Global and multinational prevalence of fungal diseases estimate precisionJ Fungi (Basel) 2017 3(4):5710.3390/jof304005729371573 [Google Scholar] [CrossRef] [PubMed]
[4]. Avila-Aguero ML, Canas-Coto A, Ulloa-Gutierrez R, Caro MA, Alfaro B, Paris MM, Risk factors for Candida infections in a neonatal intensive care unit in Costa RicaInternat J Infectio Dis 2005 9:90-95.10.1016/j.ijid.2004.05.00715708324 [Google Scholar] [CrossRef] [PubMed]
[5]. Ali GY, Algohary EH, Rashed KA, Almoghanum M, Khalifa AA, Prevalence of Candida colonisation in preterm newborns and VLBW in neonatal intensive care unit: Role of maternal colonisation as a risk factor in transmission of diseaseJ Matern Fetal Neonatal Med 2012 25(6):789-95.10.3109/14767058.2011.62200521919548 [Google Scholar] [CrossRef] [PubMed]
[6]. Shettigar CG, Shettigar S, Non albicans Candidemia: An emerging menace in neonatal intensive care unitInt J Contemp Pediatr 2018 5(2):436-41.10.18203/2349-3291.ijcp20180531 [Google Scholar] [CrossRef]
[7]. Nazir A, Masoodi T, Spectrum of candidal species isolated from neonates admitted in an Intensive Care Unit of teaching hospital of Kashmir, North IndiaJ Lab Physicians 2018 10(03):255-59.10.4103/JLP.JLP_1_1830078958 [Google Scholar] [CrossRef] [PubMed]
[8]. Azim A, Ahmed A, Baronia A, Yadav R, Sharma P, Marak R, Epidemiology of Candida colonisation in medical surgical intensive care unit of a tertiary care teaching hospital of North IndiaJ Microbiol Infectio Dis 2018 8(4):147-52.10.5799/jmid.493851 [Google Scholar] [CrossRef]
[9]. Latha GS, Veeresh Babu DV, Thejraj HK, Clinical profile of candidiasis in neonatesInt J Contemp Pediatr 2017 4(5):1875-80.10.18203/2349-3291.ijcp20173803 [Google Scholar] [CrossRef]
[10]. Pandita N, Peshin C, Wasim S, Bhat NK, Gupta A, Profile of fungal septicaemia in new born at a tertiary care hospital in North IndiaInt J Contemp Pediatr 2017 4(2):455-59.10.18203/2349-3291.ijcp20170688 [Google Scholar] [CrossRef]
[11]. Neves RP, de Carvalho Parahym AMR, da Silva CM, Macêdo DPC, Leal AFG, Neves HJ, Fungal infections in neonatal intensive careSelected Topics in Neonatal Care 2018 8:109-21.10.5772/intechopen.70302 [Google Scholar] [CrossRef]
[12]. Bansiwal K, Binnani A, Gupta PS, Study of candida colonisation and candidemia in neonates admitted in neonatal intensive care unit at a tertiary care institute of Bikaner, Rajasthan, IndiaInt J Curr Microbiol App Sci 2016 5(10):156-65.10.20546/ijcmas.2016.510.018 [Google Scholar] [CrossRef]
[13]. Abdallah Y, Kaddu-Mulindwa D, Nankunda J, Musoke PM, Prevalence and immediate outcome of Candida colonised preterm neonates admitted to Special Care Unit of Mulago Hospital, Kampala UgandaAfr Health Sci 2015 15(1):197-205.10.4314/ahs.v15i1.2625834549 [Google Scholar] [CrossRef] [PubMed]
[14]. Mahmoudabadi AZ, Rezaei-Matehkolaei A, Ghanavati F, The susceptibility patterns of Candida species isolated from urine samples to posaconazole and caspofunginJundishapur J Microbiol 2015 8(3):e2429810.5812/jjm.24298 [Google Scholar] [CrossRef]
[15]. Wadile RG, Bhate VM, Study of clinical spectrum and risk factors of neonatal candidemiaIndian J Pathol Microbiol 2015 58:472-74.10.4103/0377-4929.16888826549069 [Google Scholar] [CrossRef] [PubMed]
[16]. Noori Sanami M, Radfar M, Shirvani F, Nabavi M, Gachkar L, Candida colonisation in low birth weight and very low birth weight infants in a neonatal intensive care unitArch Pediatr Infect Dis 2015 3(4):e2123410.5812/pedinfect.21234 [Google Scholar] [CrossRef]
[17]. Tak V, Mathur P, Varghese P, Gunjiyal J, Xess I, Misra MC, The epidemiological profile of candidemia at an Indian trauma care centerJ Lab Physicians 2014 6(2):96-101.10.4103/0974-2727.14150625328334 [Google Scholar] [CrossRef] [PubMed]
[18]. Sardana V, Pandey A, Madan M, Goel SP, Asthana AK, Neonatal candidemia: A changing trendIndian J Pathol Microbiol 2012 55(1):132-33.10.4103/0377-4929.9490022499329 [Google Scholar] [CrossRef] [PubMed]
[19]. Santhanam S, Arun S, Rebekah G, Ponmudi NJ, Chandran J, Jose R, Perinatal risk factors for neonatal early-onset group b streptococcal sepsis after initiation of risk-based maternal intrapartum antibiotic prophylaxis—A case control studyJ Trop Pediatr 2018 64(4):312-16.10.1093/tropej/fmx06829036682 [Google Scholar] [CrossRef] [PubMed]
[20]. Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N, Emergence of non-albicans Candida species in neonatal candidemiaN Am J Med Sci 2013 5(9):541-45.10.4103/1947-2714.11891924251272 [Google Scholar] [CrossRef] [PubMed]