Spinal dysraphism is due to nonclosure of the neural groove, consisting of combination of malformations called spina bifida, involving ectoderm, mesoderm and neuroectoderm [1]. Combined malformations of vertebral column and spinal cord results in formation of spinal dysraphism [2]. There were different classifications of spinal dysraphism. Most of classifications are based on specific derangement of embryonic development. Spina bifida classified into many types [3]: 1) spina bifida cystica; 2) Spina bifida aperta; 3) Spina bifida occulta.
Spina bifida cystica refers to either MMC or MC. MC involves herniation of dura and arachnoid through the defect in vertebral lamina and spinal cord remain within spinal canal [2,3]. In MMCs along with herniation of dura and arachnoid, spinal cord and nerve roots become the components of herniated cystic structure. Spina bifida aperta means spinal dysraphic conditions which are communicating with the environment [4]. Spina bifida occulta is concealed form of spinal dysraphism. In recent classification of spinal dysraphism which is by Totori-Donati P et al., every lesion has been linked with embryological aspects, along with clinico-neuro radiological correlation [3,4].
Essentially spinal dysraphism divided into two main categories, these are Open Spinal Dysraphism (OSD) and Closed Spinal Dysraphism (CSD). OSD comprises MMC, myelocele, hemimyelomeningocele and meningomyelocele. CSD consists of unenclosed vertebral arches with or without externally visible cystic lesion in the back these include lipomyeloschisis, lipomyelomeningocele, MC and terminal myelocystocele [3-5]. Histopathological aspects are important in better understanding of these anomalies. Objectives of this study were to review the histopathological findings, to elucidate the ectodermal, mesodermal and neuroectodermal changes in spinal dysraphism.
Materials and Methods
A descriptive study was conducted on histopathology of spinal dysraphism cases during the period from October 2012 to February 2016 in Department of Pathology of a tertiary care hospital, Hyderabad, India. During study period, tissue specimens from total 45 cases of spinal dysraphism were referred to Pathology Department.
Inclusion criteria: Cases which were diagnosed as spinal dysraphism by clinical and radiological examination and underwent surgery for the same were included in the study.
Exclusion criteria: Cases which were diagnosed as spinal dysraphism by clinical and radiological examination and did not underwent surgery were excluded in this study.
After surgical excision, tissue specimens were sent to department of pathology along with clinical and radiological findings such as patient’s age, sex, site of lesion and macroscopic definition and radiological diagnosis. In all cases tissue specimens were immediately fixed in formalin, followed by routine tissue processing. After paraffin block preparation, 4 μm thick sections of formalin fixed, paraffin embedded material was obtained which were stained with H&E. Detailed microscopic study of H&E slides was done. Special stain Masson trichrome was performed to demonstrate fibrosis. Histopathology findings such as epithelial, mesodermal, neuroectodermal changes were elucidated in detail and findings were compared with literature.
Statistical Analysis
Statistical analysis was carried out by using Microsoft Excel and Epi info software. Data was entered in MS excel and analysed by Epi info software to obtain the percentages of different histopathological features in cases of spinal dysraphism.
Results
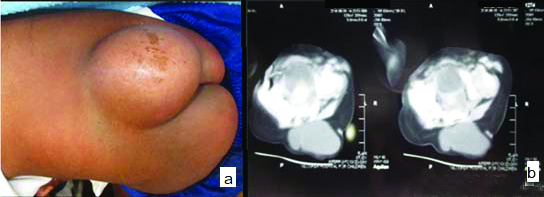
In total 45 cases of spinal dysraphism, there were 30 cases of MMC these were belonging to OSD accounting for 66.7% cases and nine cases of MC and six cases of encephalocele, which were belonging to CSD accounting for 33.3%. Among 45 cases, there were 25 females and 20 males. Age of patients was ranging from newborn to one year. All cases had cystic lesion in back. MC cases presented with skin covered protrusion of a spinal fluid filled sac of meninges through a bony defect in the posterior elements of spine [Table/Fig-1a,b].
Meningocele (MC): (a) skin covered cystic lesion in lumbosacral region; (b) CT image showing herniation of Cerebrospinal Fluid (CSF) filled sac through osseous defect of posterior spinal elements.
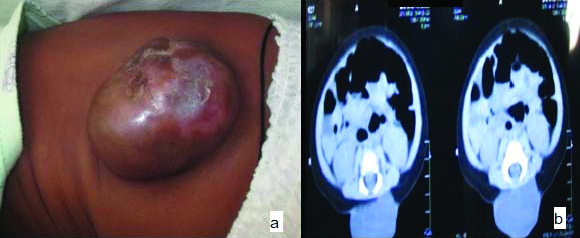
Out of 30 cases of MMC two cases were thoracolumbar, one cervical, one sacral, 26 were in lumbosacral region. In MMC cases meningeal sac herniated through a bone defect of the vertebral arch and the meningeal cystic sac contains cerebrospinal fluid, nerve roots and spinal cord. Both spinal cord, meninges are damaged and displaced through the bony defect and presented a clear open defect in the lumbar-sacral region [Table/Fig-2a,b]. In nine cases of MC seven were in lumbosacral region and two in cervical region. Out of six cases of encephalocele, all were located in the occipital region. In 45 cases 26 cases of MMC and two cases of MC were associated with hydrocephalus and two cases of MMC had club foot deformity [Table/Fig-3] showing cut section of MMC.
Myelomeningocele (MMC): (a) Cystic lesion in lumbar region covered by membranous sac; (b) CT- image showing herniation of spinal cord, meninges and well defined fluid filled lesion representing leakage of CSF through a defect in posterior lumbar spine.
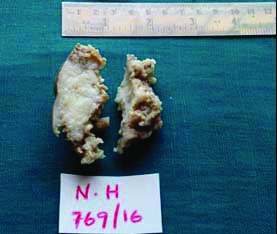
Gross finding from a case of MMC-partially skin covered grey white soft tissue mass measuring about 2 cm in its greatest diameter.
Histopathology Findings
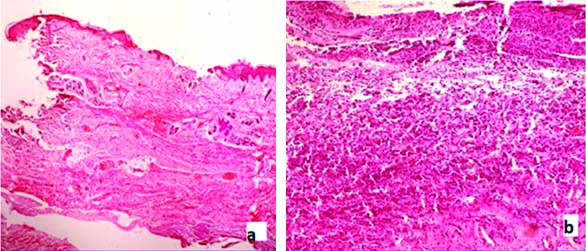
Epithelial changes- In total 45 cases, epidermis was present in 43 cases. In 25 cases ulceration along with inflammatory infiltrate [Table/Fig-4a] was noted. Loss of epidermal appendages seen in 41 cases that is in 29 cases of MMC (96%) [Table/Fig-4b], eight cases of MC (89%) and four cases of encephalocele.
a) H&E (4X)-section from a case of MMC showing epidermal ulceration; b) H&E (4X) Loss of epidermal appendages in MMC.
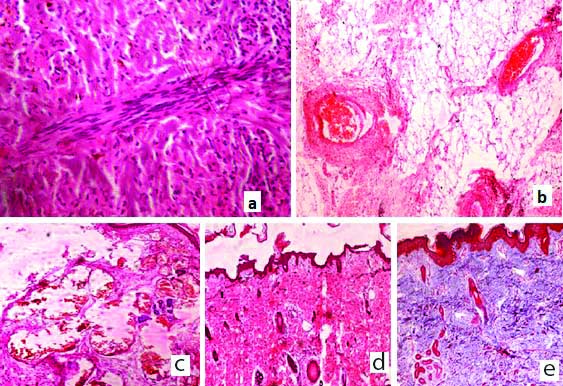
Mesodermal changes- Out of 45 cases, bundles of smooth muscle fibres [Table/Fig-5a] were found in 12 cases. Mature adipose tissue [Table/Fig-5b] was seen in 67% of total cases. In nine cases of MMC, fatty tissue was attached to the spinal cord and these were diagnosed as lipomyelomeningocele. Abnormal ectatic blood vessels [Table/Fig-5c] were seen in 35 cases. Fibrosis was seen in 40 cases (89%), sub-epithelial oedema seen in five cases. In 21 cases, bands of dense collagen fibres [Table/Fig-5d] were seen. Fibrosis was demonstrated by using Masson trichrome stain and collagen fibres were stained blue [Table/Fig-5e].
a) H&E (40X) Section from a case of MMC showing bundles of smooth muscle; b) H&E (40X) Mature adipose tissue in section from a case of MMC; c) H&E (40X) Abnormally increased and ectatic blood vessels in section from a case of MMC; d) H&E (10X) Fibrosis with dense collagen fibres in case of MC; e) Masson trichrome stain (10X) to demonstrate fibrosis in case of MC.
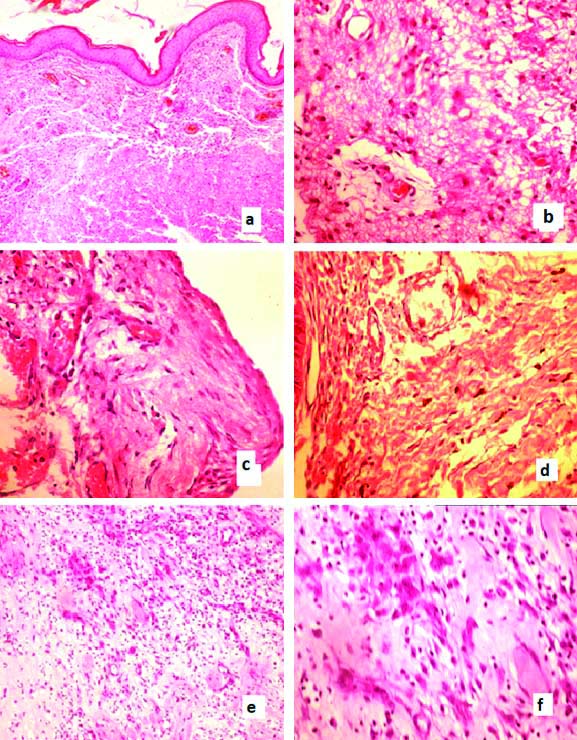
Neuroectodermal changes- Neuropil like matrix was seen in 34 cases (76%) [Table/Fig-6a,b]. Meningothelial cells [Table/Fig-6c] were noted in 28 cases (62%), nerve fibres seen in total 33 cases (76%) [Table/Fig-6d]. Cortical dysplasia seen in two cases of encephalocele [Table/Fig-6e,f]. Neuropillike matrix and nerve fibres noted in all cases of MMC and these were not found in MC cases.
a) H&E (10X) Neuropil like matrix beneath the epidermis; b) H&E (40X) Neuropil like matrix; c) H&E (40X) Meningothelial cells in section from a case of MC; d) H&E (40X) Nerve bundles in section from a case of MMC; e) H&E (4X) Cortical dysplasia in encephalocele; (f) H&E (40X) Cortical dysplasia in encephalocele.
Histopathological feature- Epithelial changes, mesodermal changes and neuroectodermal changes are summarised in [Table/Fig-7].
Summary of histopathological findings.
| Histopathological features | Total cases (n=45) (%) | MMC n=30 (%) | MC n=9 (%) | Encephalocele n=6 (%) |
|---|
| Loss of epidermal appendages | 41 (91) | 29 (96) | 8 (89) | 4 (67) |
| Ulceration | 25 (56) | 24 (80) | 1 (11) | -- |
| Inflammation | 38 (84) | 28 (93) | 6 (67) | 4 (67) |
| Fibrosis | 40 (89) | 28 (94) | 8 (89) | 4 (67) |
| Peripheral nerve fibres/roots | 33 (73) | 30 (100) | -- | 3 (50) |
| Abnormal/incr. vessels | 35 (78) | 28 (94) | 5 (56) | 2 (34) |
| Mature adipose tissue | 30 (67) | 22 (73) | 5 (55) | 3 (50) |
| Neuropil-like matrix | 34 (76) | 30 (100) | -- | 4 (67) |
| Meningothelial cells | 28 (62) | 20 (67) | 7 (78) | 1 (17) |
| ArrectorPili hyperplasia | 13 (29) | 8 (27) | 4 (45) | 1 (17) |
| Skeletal muscle | 4 (9) | 3 (10) | 1 (11) | -- |
| Subepithelial oedema | 5 (11) | 4 (13) | 1 (11) | - |
| Cortical dysplasia | 2 (4) | -- | --- | 2 (34) |
Discussion
Spinal dysraphism is most common congenital anomaly of central nervous system which is due to defect in closure of neural tube at caudal neuropore during embryonic period [7,8], such congenital malformations are categorised as Neural Tube Defects (NTDs), mainly classified into open defects such as craniorachischisis, anencephaly and MMC and closed defects such as encephalocele, MC and spina bifida occulta. The overall estimated prevalence of spinal dysraphism is around one in 1000 births, with regional and ethnic variations [9]. MMC is most common form of spina bifida and it is associated with significant lifelong morbidity. Cases of spina bifida are associated with motor and sensory deficits, apart from this, significant complications will arise from hydrocephalus and Arnold chairi II malformation. In the present study, 26 cases of MMC were associated with hydrocephalus; two cases are associated with Arnold chairi II malformation.
Myelomeningocele is usually associated with hydrocephalus and Arnold Chiari malformation. Traction of the brain stem from below due to tethering of the open spinal cord through the vertebral defect leads to Arnold chairi II malformation, because of that brain stem is elongated, the cerebellar vermis is displaced into the foramen magnum and therefore the normal flow of Cerebrospinal Fluid (CSF) through the ventricles is compromised causing hydrocephalus [10].
Neurological deficits include motor, sensory and sphincter dysfunction, depending upon the severity and level. In severe cases, hypotonia of the limbs, sphincter atony with rectal prolapse may be associated. Arnold Chiari malformation presents with lower brainstem and lower cranial nerve dysfunction. Kyphosis, scoliosis, and deformities of the long bones and feet, hemivertebrae, defective ribs are most common associated skeletal abnormalities [10]. Apart from skeletal abnormalities, spina bifida may be associated with facial clefts, genitourinary abnormalities and cardiac abnormalities such as dextrocardia [11].
At birth, spina bifida is more common in girls than in boys. In the present study, F/M Ratio was 1.4 to 1, majority of MMC are lumbosacral in location. Cervical or cervical-thoracic locations of MMCs are rare. Almost 1% of the cases show multi segmental involvement. In present study, among 30 MMCs, 26 were lumbosacral, two thoraco-lumbar one in sacral and one was in the cervical region. All Cases of encephalocele in present study were seen in the occipital region. Encephaloceles are rare congenital anomalies. Encephalocele cases characterised by defects in skull with partial lacking of bone fusion leaving a gap through which a portion of brain and meninges protrude, it may be located at any part of head, but most often affects base of skull (occipital area) [6,12].
To understand the pathological features of spina bifida, brief review of normal embryology of involved structures is essential. Development of CNS involves two different processes- primary neurulation and secondary neurulation. Primary neurulation occurs in third week of intrauterine life, during this process notochord induces the overlying ectoderm to differentiate into specialised neural plate, later neural folds are formed by elevation of lateral edges of neural plate, it is followed by continuous elevation of neural folds and they approach each other in midline, then fuse to form a neural tube. This process of fusion and formation of neural tube begins in cervical region and proceeds in both cephalic and caudal directions [12].
Secondary neurulation involves caudal cell mass development which forms caudal most portion of neural tube and it forms spinal segments below L-2. Neural tube closure is followed by the process of dysjunction during which, the epithelial ectoderm separates from neural ectoderm, later fusion of epithelial layers give rise to skin covering the neural tube. The mesenchyme will form meninges, neural arches of vertebrae and paraspinal muscles, by migrating between neural tube and skin. Spinal cord extends entire length of fetus by third month of intrauterine life. As the development continues terminal end of spinal cord shifts to higher vertebral level due to rapid lengthening of vertebral column and dura than the neural tube [12].
Most cases of spinal dysraphism both OSD and CSD result from embryological abnormalities during the process of primary neurulation, few cases arise from premature disjunction which leads to fusion of the spinal cord with fatty elements. In 91% of cases we noted diminution or complete loss of epidermal appendages. Karabagli P et al., reported loss or decrease in appendageal structures in 95% of cases [5]. Berry AD and Patterson JW also reported loss of adnexal structures in 72% of cases of spinal dysraphism [Table/Fig-8]. Most of cases of MMC shows characteristic central area of loss of epithelial lining or ulceration [1], leads to exposure of vascular tissue from which there is oozing of CSF or tissue transudate occurs [13]. In the present study, epithelial ulceration noted in 56% of cases and abnormally increased number of ectatic thin walled blood vessels were noted in 78% of cases. Vascular proliferation which resembled angioma as seen in two MMC cases but because of presence of other tissue elements like meningothelial cells and spinal nerve roots, considered as MMC with cutaneous haemangioma. In MMC, one of superficial skin manifestation is cutaneous haemangioma [14].
Showing comparison of histopathological findings with other similar studies.
| Histopathological features | Berry AD and Patterson JW [1] study in 132 cases (1991) | Karabagli P et al., [5] study in 43 cases (2014) | Present study in 45 cases (2021) |
|---|
| Loss of epidermal appendages | 72% | 93% | 91% |
| Fibrosis | 99% | 88% | 89% |
| Peripheral nerve fibres/roots | 48% | 83% | 73% |
| Abnormal/incr. vessels | 83% | 79% | 78% |
| Neuropil-like matrix | 71% | 79% | 76% |
| Ulceration | 35% | 66% | 56% |
| Mature Adipose tissue | 38% | 62% | 67% |
| Meningothelial cells | 26% | 25% | 62% |
| Arrectorpili hyperplasia | 42% | 18% | 29% |
| Skeletal muscle | 5% | 4% | 9% |
Proliferation and hypertrophy of pilar smooth muscle fibres seen in 29% of cases in present study. Karabagli P et al., have reported the same finding in 56 out of 132 cases of spinal dysraphism (42%) [5]. In study of Talwalker VC and Dastur DK on 51 cases of spinal dysraphism, three cases had increased proliferation of smooth muscle fibres among the abnormalities of mesodermal tissue [15]. Intraspinal Lipomas are one of common finding in spinal dysraphism cases. In the present study 67% of cases showed sheets of mature fat cells, which can be confused with spinal lipomatous masses. However, this could be differentiated from lipoma by looking at infiltrative margins and lack of capsule, as classic spinal lipoma will have intact capsule [16]. Prognosis is related to anatomic level of MMC [16,17]. MMC in cervical region having more favourable outcome, as cervical MMC cases are more clearly protuberant, covered with full thickness of skin at base and by thick epithelium on the dome. So, neural tissue is not exposed superficially. These factors are responsible for more favourable outcome in cervical MMC cases [16,17]. In the present study, there is one case of MMC in cervical location, and is covered by thick skin without ulceration and having a good post-surgical prognosis.
Meningocele and MMC are macroscopically very similar, but they represent two different opposite types of spina bifida, whereas MC is a closed defect and MMC is an open defect and both are having different prognosis. Presence of neural tissues in the meningeal sac (medulla and/or nerves) helps us to differentiate MMC from MC, which were absent in later [18].
Limitation(s)
The present study was limited to cases of spinal dysraphism who underwent surgical intervention.
Conclusion(s)
Congenital malformations of spine and spinal cord can be complex and variable in imaging appearance. An organised approach to imaging findings with consideration of clinical and pathological findings allows greater ease in diagnosis. The embryogenesis of spina bifida involves ectoderm, neuroectoderm and mesoderm. A detailed definition of histopathological aspects (ectodermal, mesodermal and neuroectodermal changes) in cases of spina bifida will help in understanding these anomalies and these findings should be a part of detailed histopathology report in cases of spina bifida.